Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder, with a lifetime prevalence in the United States of approximately 6% (
1). Rates vary by trauma type, severity, and cumulative trauma exposure, with the highest PTSD burden among survivors of interpersonal violence (
2). Despite its prevalence, currently available treatments for PTSD have limitations. While trauma-focused psychotherapies have the most empirical support, they are limited by significant rates of nonresponse, partial response, and treatment dropout (
3,
4). There are few available pharmacotherapies for PTSD, and their efficacy is insufficient. Only two medications—the selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine—are approved by the U.S. Food and Drug Administration (FDA) for PTSD treatment, and only four medications have shown at least moderate-level evidence of efficacy in the treatment of the disorder (
5,
6). Approximately 60% of patients with PTSD respond to SSRIs, and only 20%–30% achieve complete remission following pharmacotherapy (
7), resulting in poly‐pharmacy in clinical practice (
8,
9). Additionally, maximal improvement with available medications can take months. The lack of progress in PTSD pharmacotherapy, recently labeled a crisis (
10), has made it imperative to develop novel pharmacologic interventions for chronic PTSD.
Ketamine, first approved by the FDA as an anesthetic agent in 1970 (
11), is a noncompetitive glutamate
N-methyl-
d-aspartate (NMDA) receptor antagonist, with lower affinity for serotonin, dopamine, opioid, and other receptors (
12). After decades of use for anesthesia, analgesia, and sedation, the efficacy of single-dose intravenous ketamine infusion for treatment-resistant depression was established (
13,
14), and repeated infusions were demonstrated to prolong improvement (
15–
17). Our previous proof-of-concept randomized controlled trial (
18) of single-dose intravenous ketamine in individuals with chronic PTSD, compared with single-dose intravenous midazolam, provided the first evidence of rapid symptom reduction after ketamine infusion in patients with this disorder. Given the urgent need to investigate ketamine’s potential as a viable longer-term treatment, we sought to evaluate the efficacy of a 2-week course of repeated ketamine infusions and the durability of treatment response in individuals with chronic PTSD. We aimed to examine the effect of repeated ketamine administration on overall PTSD symptom severity, and additionally on individual symptom clusters (intrusion, avoidance, negative mood and cognitions, and arousal and reactivity) and on comorbid depressive symptoms; to evaluate the safety and tolerability of repeated infusions in PTSD patients; and to assess the durability of treatment response.
To our knowledge, this is the first randomized controlled trial of repeated ketamine administration in individuals with chronic PTSD. In this parallel-arm randomized controlled trial, the efficacy of repeated intravenous ketamine infusions was compared with that of repeated infusions of midazolam as the psychoactive control drug in a sample of individuals with PTSD from civilian or military trauma. Our primary hypothesis was that repeated ketamine infusions would be associated with significantly greater reduction in overall PTSD symptoms compared with midazolam infusions, assessed 2 weeks after the first infusion. Additionally, we hypothesized that a higher proportion of patients in the ketamine group would exhibit clinically significant improvement, defined as ≥30% reduction in symptoms, a commonly used threshold in clinical trials for patients with PTSD (
19–
21). Based on findings from our single-dose ketamine infusion randomized controlled trial (
18), we also hypothesized that ketamine infusions would be associated with significant improvements in all PTSD symptom clusters and comorbid depressive symptoms.
METHODS
The study and protocol modifications were approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (ISMMS). Written informed consent was obtained from all participants, and all participants completed an independent capacity assessment.
Study Design
This was a randomized double-blind parallel-arm controlled study conducted between June 2015 and January 2020 at the ISMMS Depression and Anxiety Center for Discovery and Treatment. The study involved three phases: 1) screening, during which participants were assessed for eligibility and any required medication tapering took place; 2) a 2-week treatment phase, during which participants received six infusions of study drug approximately three times per week and were assessed on each infusion day, as well as 24 hours and 2 weeks after the first infusion; and 3) a follow-up phase for participants who showed treatment response, defined as ≥30% symptom improvement 2 weeks after the first infusion (
19–
21), assessed with the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) (
22), using a past-week recall period (see also Figure S1 in the online supplement). Treatment responders were followed naturalistically, weekly for up to 4 weeks and then monthly until loss of responder status or up to 6 months if there was no loss of response. To assess for eligibility, a trained study rater administered the Structured Clinical Interview for DSM-5 (SCID-5) (
23) and the CAPS-5. Additionally, the principal investigator, a psychiatrist, reviewed each patient’s ratings, medical history, and concomitant medications, conducted a clinical evaluation with each patient, and administered the Columbia-Suicide Severity Rating Scale (C-SSRS) (
24) and the Clinical Global Impressions severity scale (CGI-S) (
25). All patients underwent a physical examination, basic laboratory screening, including routine hematologic, biochemical, and urine toxicology testing, and an ECG to rule out unstable medical conditions and current substance use other than marijuana.
Study Population
Participants were eligible if they were between ages 18 and 70, had a current primary diagnosis of chronic PTSD as defined by DSM-5 criteria (of at least 3 months’ duration) and determined by the SCID-5, and had a score ≥30 on the CAPS-5, which is several points into the moderate severity range. Exclusion criteria were any serious unstable medical condition, active suicidal or homicidal ideation (having a plan or inability to contract a no-harm agreement), lifetime history of psychotic or bipolar disorder, current anorexia or bulimia nervosa, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than one occasion or any use in the past 2 years, and current treatment with a long-acting benzodiazepine or opioid medication. Stable dosages of other psychotropic medications (e.g., antidepressants, antipsychotics, or mood stabilizers) for at least 3 months before randomization were permitted. For patients taking a short- acting benzodiazepine, morning doses were held on infusion days. Although individuals with marijuana use disorder were excluded, current use of marijuana or cannabis derivatives was permitted (
Table 1), given its prevalence among patients with PTSD (for further details on inclusion and exclusion criteria, see
ClinicalTrials.gov NCT02397889). Participants who were taking a contraindicated psychotropic medication that was not effective or medically necessary were given the opportunity to taper off the medication before randomization, under the supervision of their treating psychiatrist.
Randomization and Blinding
A randomization schedule was generated by the ISMMS research pharmacy, employing randomly permuted blocks, to assign participants to receive six infusions of either ketamine hydrochloride (0.5 mg/kg) or midazolam (0.045 mg/kg). Eligible participants were randomly assigned on the morning of their first infusion. All participants and study personnel were blinded to treatment assignment, which was known only to the research pharmacy. Trained raters administered safety ratings during infusions and 40 and 120 minutes after infusions. A different set of trained raters, blinded to the ratings conducted during and after infusions on infusion days, administered the CAPS-5 and the Montgomery-Åsberg Depression Rating Scale (MADRS) at baseline, at the 1-week and 2-week assessments, and during the follow-up assessments. These same raters also administered the MADRS 24 hours after the first infusion.
Study Drug Administration
Both study drugs (ketamine and midazolam) were administered intravenously using the same procedure at each infusion day. Participants were scheduled to receive a total of six infusions approximately three times per week over 2 consecutive weeks. On the morning of each infusion, participants were admitted to an outpatient research unit. After confirming that participants were nil per os, tested negative on point-of-care urine pregnancy (in females of child-bearing potential) and on toxicology tests with the exception of marijuana, and met the cutoff for baseline blood pressure (no more than two readings above 140/90 mmHg or one above 160/90 mmHg), an indwelling catheter was placed in the antecubital vein of the nondominant arm. The study drug was administered over 40 minutes via an infusion pump. Continuous ECG monitoring was conducted from infusion start to end. Pulse, blood pressure, and oxygen saturation were monitored every 5 minutes during infusion and every 15 minutes postinfusion as appropriate (for further details, see reference 14). A study physician (anesthesiologist or psychiatrist trained in sedation procedures) oversaw all infusions. A nurse monitored the patient during each infusion and up to 120 minutes after the start of the infusion. A study physician assessed all participants before their discharge from the research unit.
Efficacy Measures
The primary outcome was change in PTSD symptom severity, compared between the two groups, as measured with the CAPS-5, administered at baseline just before the first infusion, at 1 week (just before the fourth infusion), and at 2 weeks (after completion of all six infusions, usually on the Monday after the second week of infusions), with a past-week recall period. The CAPS-5 with past-week recall was additionally administered at follow-up visits. Secondary outcome measures were the CAPS-5’s four PTSD symptom clusters and the MADRS with past-week recall (
26), administered at the same time points; the Clinical Global Impressions severity (CGI-S) and improvement (CGI-I) scales, administered by a study clinician at each time point to evaluate overall clinical status at that moment; and the Impact of Event Scale–Revised (IES-R, self-report) (
27) and the MADRS, both modified for past 24-hour recall, administered at baseline and 24 hours after the first infusion.
Side Effect and Safety Measures
On infusion days, potential psychotomimetic, dissociative, and manic symptoms were assessed before infusion and at 40 and 120 minutes after start of infusion with the Clinician‐Administered Dissociative States Scale (
28), the 4-item positive symptom subscale of the Brief Psychiatric Rating Scale (
29), and the first item (elevated mood) of the Young Mania Rating Scale (
30). The Patient-Rated Inventory of Side Effects (PRISE) was used to assess side effects in eight organ systems and general side effects. Acute infusion-related side effects were assessed with the PRISE 120 minutes after start of infusion, covering the period since start of infusion. Side effects were also assessed with the PRISE before each infusion, covering the period since the previous assessment. Additionally, the C-SSRS was administered, with past-week recall.
Statistical Analysis
A priori power calculations were conducted on the basis of results from our previously published single-dose ketamine infusion randomized controlled trial (
18). In that study, which employed a crossover design, a mean decrease of 27 points (SD=20) on the CAPS (DSM-IV) total score was observed in the ketamine arm at 7 days, compared with a mean decrease of 13 points (SD=18) in the midazolam arm (d=0.74). Thus, assuming a paired two-sample t test, we calculated that a sample size of 40 would provide >80% power to detect a between-group difference of this magnitude, assuming a type I error rate of 0.025 (using the Bonferroni adjustment to hold the family-wise error rate at 0.05).
Although we had originally planned for a total sample size of 40 participants, partially unblinded interim analyses (drug A, drug B), also planned, were conducted by an independent statistician with 30 participants. For this interim analysis, an intent-to-treat mixed-effects model approach was used to compare the primary outcome measure (CAPS-5) between the two treatment groups at baseline and at 1 week and 2 weeks. From this model, adjusted means were calculated at the three time points, and two-sample t tests were used to compare the two treatment groups at each of these time points. The robust, large-magnitude, and clinically significant difference (d=1.13; 66.7% compared with 20.0% treatment responders) between the two groups led to our decision to stop the trial with 30 participants, in consultation with the study data safety monitoring board.
For all outcome measures, we utilized intent-to-treat mixed-effects model analyses with time, treatment, and their interaction, assuming compound symmetry for the correlation matrix across time points. For the CAPS-5 total, CAPS-5 subscale, and MADRS total scores with past-week recall and for the CGI-S score, the time points were baseline, 1 week, and 2 weeks. Time points for the CGI-I score were 1 and 2 weeks, and for the IES-R and MADRS with past-24-hour recall, they were baseline and 24 hours after the first infusion. A chi-square test was conducted to compare percentages of responders in the two treatment groups. We produced Kaplan-Meier plots for probability of loss of response by treatment, with right-censoring for those who did not lose response. All analyses were conducted at an alpha of 0.0304 per the Pocock alpha spending function and in SAS, version 9.4, using linear mixed models (PROC MIXED) and cumulative logit mixed models (PROC GLIMMIX) for ana- lyses of CGI-S and CGI-I scores.
RESULTS
Of the 51 potential participants who completed informed consent procedures, 30 met eligibility criteria and were randomly assigned to receive ketamine or midazolam infusions (
Figure 1). The vast majority of participants (N=29, 97%) completed all six infusions. One participant was withdrawn from the study per protocol after the first two infusions because of lack of improvement and concern about worsening of PTSD symptoms.
The participants’ demographic and clinical characteristics are summarized in
Table 1. The mean PTSD duration for the full sample was 14.9 years (SD=13.5), with mean symptom levels in the severe range at study enrollment. Almost half the sample reported sexual assault or molestation as their primary or index trauma, followed by physical assault or abuse, witnessing violent assault or death, having survived or responded to the terrorist attacks of September 11, 2001, and combat exposure. Thirteen (43.3%) participants were on stable dosages of concomitant psychotropic medications, and 17 (56.7%) participants were receiving concomitant psychotherapy (see Table S1 in the online supplement). Only two (6.7%) of 30 participants who underwent randomization required a medication taper or change before randomization. The participants’ prior treatment history is summarized in Table S2 in the online supplement.
Efficacy Results
PTSD symptom severity.
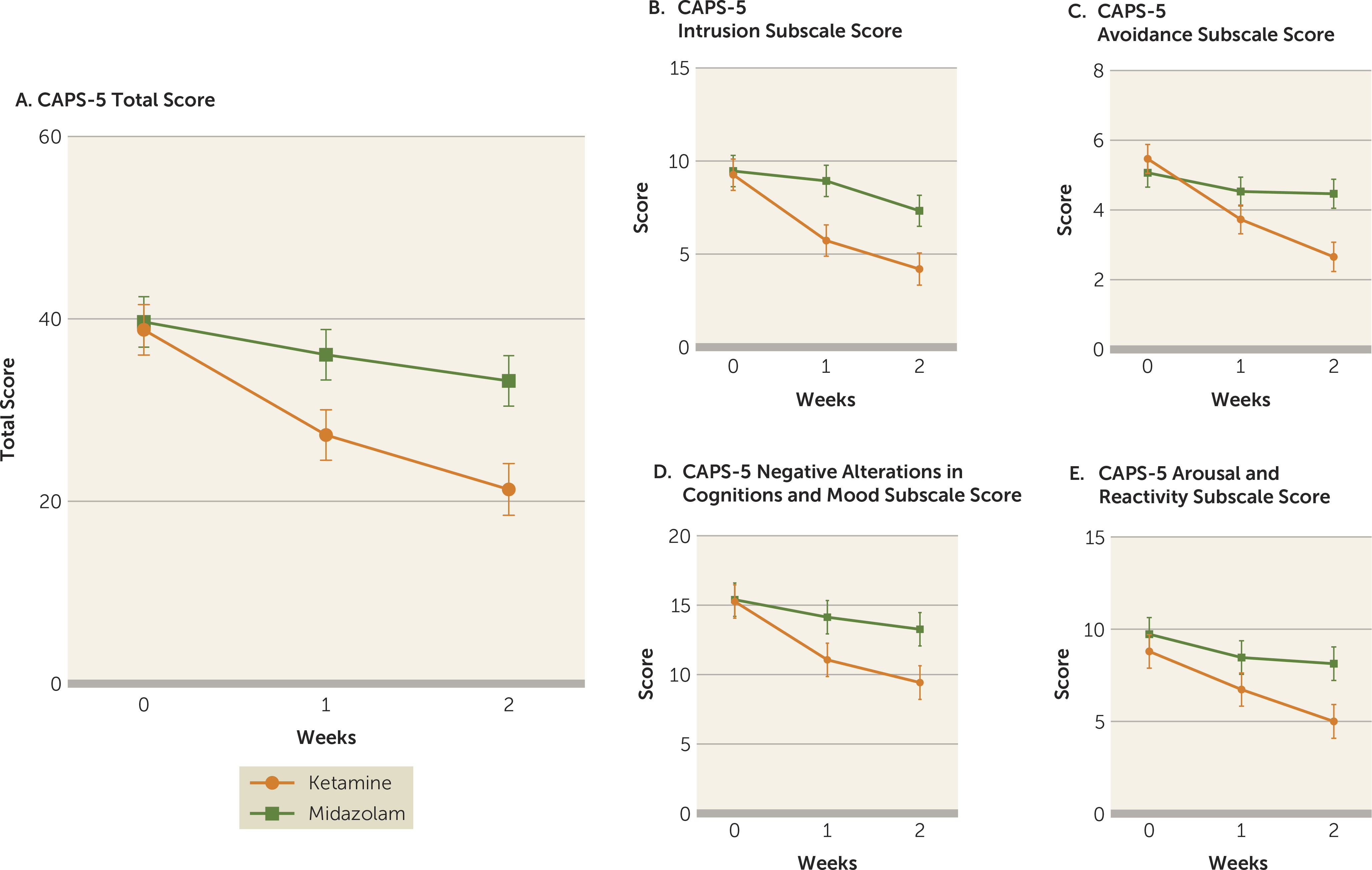
The mixed-effects model analysis with total CAPS-5 scores as the dependent variable revealed a significant treatment-by-time interaction (F=5.97, df=2, 55, p=0.0045). Improvement in total CAPS-5 scores over the course of the study visits (baseline, week 1, and week 2) differed significantly between the ketamine and midazolam treatment groups. As shown in
Figure 2A, from comparison of the adjusted means, while CAPS-5 scores were similar in both treatment groups at baseline (estimated difference=0.87, SE=3.93, p=0.83), total CAPS-5 scores were significantly lower in the ketamine group at week 1 (estimated difference=8.80, SE=3.93, p=0.030) and at week 2 (estimated difference=11.88, SE=3.96, p=0.004) compared with the midazolam group (effect size at week 1: d=0.85, 95% CI=0.10, 1.59; at week 2: d=1.13, 95% CI=0.36, 1.91) (see also Table S3 in the online supplement).
Additionally, significantly more participants in the ketamine group (N=10/15, 67%) attained response (≥30% reduction from baseline) at week 2 compared with those in the midazolam group (N=3/15, 20%; χ
2=4.89, p=0.03), with a number needed to treat of 2.1, suggesting that 2.1 additional patients with PTSD would need to be treated with ketamine (compared with midazolam) to attain one additional response. In separate mixed-model analyses exploring the effect of ketamine compared with midazolam on each of the four CAPS-5 subscales, there was a significant treatment-by-time interaction for three symptom clusters—intrusion (F=3.77, df=2, 55, p=0.03), avoidance (F=12.29, df=2, 55, p<0.0001), and negative mood and cognitions (F=4.42, df=2, 55, p=0.02) (
Figure 2B–D)—but not for the fourth symptom cluster, arousal and reactivity (F=2.47, df=2, 55, p=0.09) (
Figure 2E; see also Table S3 in the online supplement).
Depressive symptom severity.
A mixed-effects model analysis with total past-week MADRS scores as the dependent variable revealed a significant treatment-by-time interaction (F=5.68, df=2, 55, p=0.006, d=0.92). Improvement over study visits (baseline, week 1, and week 2) was significantly greater in the ketamine group (
Figure 3).
Clinical Global Impressions.
Separate mixed-effects model analyses with CGI-S (baseline, week 1, and week 2) and CGI-I (week 1 and week 2) scores as dependent variables revealed a significant treatment-by-time interaction on the CGI-S score (F=5.19, df=2, 50, p=0.009) and a significant main effect of treatment on the CGI-I score (F=9.96, df=1, 24, p=0.004), indicating greater clinical improvement in the ketamine group. (For selected comments quoted from ketamine responders, see Box S1 in the online supplement; for integrity of the blind, see Box S2.)
Changes in PTSD and depressive symptoms from baseline to 24 hours after the first infusion.
Separate mixed-model analyses examining symptom improvement from baseline to 24 hours after the first infusion showed no significant differences in the total IES-R score (F=0.08, df=1, 28, p=0.78) or past-24-hour MADRS score (F=0.50, df=1, 28, p=0.48). Of note, the mean change from baseline to 24 hours in the subsample of 10 ketamine responders was --26.0 on the total IES-R score, a 63.3% improvement on average. Similarly, the mean change from baseline to 24 hours in the subsample of ketamine responders was --13.4 on the total past-24-hour MADRS score, a 53.3% improvement on average. These findings suggest that improvements in both PTSD and comorbid depressive symptoms were rapid when present (see also Figures S2 and S3 in the online supplement).
Safety and side effects.
Dissociative symptoms that emerged during ketamine infusions were transient, with the highest levels reported at the infusion-end assessment (40 minutes), and had resolved by the next assessment, 120 minutes after start of infusion. No significant psychotic or manic symptoms were observed (see Figure S4 in the online supplement). One patient required acute treatment (labetalol, 5 mg intravenous) for elevated blood pressure during the fifth infusion. The most frequently reported general side effects of ketamine (compared with midazolam) recorded using the PRISE after the start of infusions included blurred vision (53% compared with 0%), dizziness (33% compared with 13%), fatigue (33% compared with 87%), headache (27% compared with 13%), and nausea or vomiting (20% compared with 7%) (see Table S4 in the online supplement).
The most common side effects of ketamine (compared with midazolam) recorded with the PRISE at other time points (before the start of infusions, 24 hours after the first infusion, and on the primary outcome assessment day) covering the interval since the prior assessment included nausea or vomiting (33% compared with 20%), headache (33% compared with 20%), and fatigue (20% compared with 7%) (see Table S5 in the online supplement). On the past-week C-SSRS at baseline, eight (53.3%) patients in the ketamine group and two (13.3%) in the midazolam group answered positively on at least one question. Among these patients, six of the eight (75%) in the ketamine group and one of the two (50.0%) in the midazolam group showed improvement on the past-week C-SSRS score at 2 weeks (see Table S6 in the online supplement). No suicidal behavior was present during the assessment period.
Risk of loss of response following response to ketamine.
Ketamine responders were followed naturalistically, initially weekly and then monthly until loss of response. Among responders to ketamine, the median time to loss of response was 27.5 days after the primary outcome assessment day, and the 25th and 75th percentiles were 23 and 32 days, respectively. Two participants had not lost response by their last assessments, which occurred 50 and 102 days after their primary outcome assessment, respectively (
Figure 4).
DISCUSSION
In this randomized controlled trial in individuals with chronic PTSD, repeated intravenous ketamine infusions administered over 2 weeks were associated with a large-magnitude, clinically significant improvement in PTSD symptoms compared with repeated midazolam infusions, a psychoactive placebo control medication. To our knowledge, these findings provide the first evidence from a randomized controlled trial of the efficacy of repeated ketamine administration for PTSD symptoms in individuals with chronic PTSD. Ketamine infusions were associated with marked improvements across three of the four PTSD symptom clusters: intrusions, avoidance, and negative alterations in cognitions and mood. In the subsample of ketamine responders, improvement in PTSD symptoms was rapid, observed 24 hours after the first infusion, and maintained for a median of 27.5 days after the primary outcome assessment day.
Individuals in this study had, on average, severe and chronic PTSD, with a median duration of 15 years, and nearly half of the sample were taking concomitant psychotropic medications. In addition to PTSD symptom improvement, the ketamine group exhibited markedly greater reduction in comorbid depressive symptoms compared with the midazolam group, which is noteworthy given the high comorbidity of depression among individuals with PTSD (
1). Large-magnitude improvements were also observed on clinical ratings of global psychiatric illness severity. The study findings further suggest that repeated ketamine infusions are safe and generally well tolerated among individuals with chronic PTSD, with only transient emergence of psychoactive and hemodynamic side effects.
Strengths of this study include the repeated-infusion design, providing insight into longer-term treatment efficacy of ketamine for PTSD; the use of a psychoactive placebo control medication; the use of both clinician-administered and self-report measures; and the inclusion of patients taking concomitant psychotropic medications. Midazolam is also an anxiolytic drug. Although benzodiazepines have not shown beneficial effects in the few studies conducted with PTSD patients (
31), midazolam could have induced transient benefits in some patients, potentially raising the bar for dem- onstrating the efficacy of ketamine for PTSD and possibly underlying the absence of between-group differences in symptom severity 24 hours after the first infusion. Of note, in an open-label trial of repeated ketamine infusions in patients with comorbid treatment-resistant depression and chronic PTSD, improvement was observed across all PTSD symptom clusters (
32). It is possible that in our randomized controlled trial, the effect of midazolam on arousal and reactivity symptoms explains the lack of a significant between-group difference for that symptom cluster.
Although the biological mechanisms underlying response to ketamine in patients with PTSD are unknown, potential mechanisms of action are suggested by findings from preclinical research and studies of patients with treatment-resistant depression. Animal studies have demonstrated that ketamine at subanesthetic doses causes a rapid increase in glutamate release, triggering several downstream signaling pathways (including brain-derived neurotrophic factor [BDNF] and mammalian target of rapamycin complex 1 [mTORC1]), resulting in increased synaptogenesis and synaptic spine maturation in key brain regions (
33,
34). Single-dose intraperitoneal ketamine administered to rats after 3 weeks of chronic unpredictable stress rapidly reversed the behavioral and biological consequences of chronic stress (
35). Additionally, in animal models of PTSD, ketamine triggered changes in BDNF levels in the hippocampus and prefrontal cortex (
36,
37). It has been proposed that persistent and recurrent symptoms experienced by individuals with PTSD, often triggered by trauma reminders, may be akin to chronic stress, associated with disruptions in glutamate signaling and connectivity in cortico-limbic circuitry. Based on ketamine studies in animal models of chronic stress and PTSD, it is hypothesized that these disruptions may be reversed by ketamine (
38,
39). While neuroimaging studies of individuals with PTSD after ketamine treatment are currently in progress, ketamine has been found to normalize dysconnectivity of the prefrontal cortex and default mode network with other brain regions in patients with major depressive disorder (
40,
41).
Among the limitations of this study is the exclusion of patients with comorbid psychotic, bipolar, or current alcohol or substance use disorders in order to protect against worsening of psychotic symptoms or abuse potential, which may limit the generalizability of our findings. Patients with active suicidal ideation were also excluded, because examining the efficacy of repeated ketamine administration in reducing suicidality in patients with PTSD was beyond the scope of this study. Other limitations include the relatively small sample size, as well as the higher rate of transient dissociative symptoms in the ketamine group potentially affecting the blind. Although three participants in the ketamine group used marijuana, only one of these three continued to use marijuana during the 2-week treatment phase, and the other two resumed use only after the primary outcome assessment day, during the follow-up phase. Finally, although use of concomitant psychotropic medications was permitted, this study was not designed to evaluate the efficacy of repeated ketamine infusions in patients with treatment-resistant PTSD, which should be examined in future studies. Of note, intranasal esketamine, the S-enantiomer of ketamine, is FDA approved as an adjunctive treatment for patients with treatment-resistant depression and also for the treatment of depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Research is needed to determine the efficacy of esketamine for PTSD treatment.
Notwithstanding these limitations, the results of this first known randomized controlled trial of repeated ketamine administration in individuals with chronic and severe PTSD revealed large-magnitude improvements in PTSD symptoms, comorbid depressive symptoms, and global illness severity after a 2-week course of ketamine infusions, with PTSD symptom improvement maintained for a median of 27.5 days following the course of infusions. The study findings suggest the overall safety and tolerability of repeated ketamine infusions in this patient population. Further research is needed to evaluate the efficacy of novel interventions to prevent PTSD symptom relapse over time, including clinical trials to evaluate the safety, tolerability, and efficacy of periodic intravenous ketamine (or intranasal esketamine) administration as maintenance over several months, which has been studied in patients with treatment-resistant depression (
42), and studies examining whether combining repeated ketamine administration with evidence-based psychotherapy for PTSD can help reduce the likelihood of relapse after cessation of ketamine infusions.
Acknowledgments
The authors thank the research pharmacists and the Clinical Research Unit and Psychiatry Department nursing personnel at ISMMS for their extensive work on this research. The authors also thank the members of the Mount Sinai Ketamine Data and Safety Monitoring Board/Ketamine Oversight Committee for their assistance and study oversight, as well as all of the patients who volunteered to participate in the study.