Autistic spectrum disorders (ASD), including autism and Asperger syndrome, are characterized by social impairments; communication difficulties; and restricted, repetitive, and stereotyped patterns of behavior. Beyond these core features, empirical and anecdotal evidence has suggested that a broader array of symptoms may be seen. Among them, motor dysfunction has been discussed. Nevertheless, further investigation and description of this domain in relation to ASDs is needed. In fact, although evaluation of sensorimotor integrity is often overlooked empirically, it remains an established means of determining the integrity of the CNS and is even predictive of higher cognitive functioning.
1 The present study was approached with two purposes: first, to examine the emerging motor profiles of individuals with autism and Asperger syndrome by way of a comprehensive and standardized measure; second, to evaluate whether inclusion of motor data adds to diagnostic clarity.
Motor Impairment and ASDs
Discrepancies between autism and Asperger syndrome in language and communication skills remain the most conservative means of differential diagnosis. However, emerging research has suggested that discrepancies stretch well beyond this realm, indicating a broader differentiation of the two, which is likely attributed to differences in underlying neurology. For example, Basnet and colleagues
2 recently demonstrated that even after statistically controlling for differences between these groups in language, neuropsychological discrepancies remain. We were interested in whether motor functioning would also represent an area of differentiation.
Consideration of motor abnormalities within these groups is not a novel concept. Although motor abnormalities are not diagnostic features of pervasive developmental disorders, including ASDs, they have been commonly described in the literature. Abnormalities of posture and clumsiness and awkwardness have been discussed in relation to autism and Asperger's, respectively, within the literature. Children with Asperger syndrome, as an example, usually have a history of developmental delays in motor skills such as pedaling a bike, catching a ball, or climbing outdoor play equipment. They are often awkward and poorly coordinated, with a walk that can appear either stilted or bouncy.
3 In the case of both autism and Asperger syndrome, although they are often diagnosed during toddler, preschool, or early-childhood years, retrospective studies using video analysis have suggested that motor signs may be apparent as early as the first year of life.
4 These same researchers have suggested that fine-motor delays are among the earliest identifiable signs distinguishing infants with autism from their peers. Others have suggested that both groups demonstrate a similar global motor delay. Both autism and Asperger's have been associated with general dyscoordination and a “lack of motor smoothness.”
5 These global deficits may stem from a multitude of factors, including, but not limited to, motor planning, fluidity, and sensory feedback.
When a simple motor-reprogramming task was used, it indicated that both individuals with autism and Asperger disorder had atypical movement preparation, with an intact ability to execute movement. An atypical deficit in motor-preparation was found in Asperger disorder, whereas movement-preparation was characterized by a “lack of anticipation” in autism.
6 Using the Bruininks-Oseretsky test to assess clumsiness, both the AS and HFA (high-functioning autism) groups showed problems with coordination, and the distribution of standard scores was virtually identical. This suggests that motor clumsiness, as measured by tests of coordination, may not reliably distinguish AS from HFA.
7 Asperger subjects have also demonstrated 1) decreased pointing accuracy and rate; 2) increased postural instability; and 3) decreased timing accuracy.
8 This may reflect impairment in the ability to integrate sensory input with motor commands, consistent with cerebellar dysfunction in Asperger's syndrome.
Association of Motor Integrity and General Functioning
Interest in motor dysfunction within these groups stretches beyond the purely investigational realm. There is a translational component that may carry over into general functioning. For example, deficits in functional independence in activities of daily living has been linked with atypical sensory responses and motor difficulties, especially fine-motor difficulties, within these groupings.
9 This link may be developmental in nature, as motor skills and sensory integration provide the means by which children first explore and make sense of the world around them. It is because of this that Piaget himself, when discussing cognitive development, termed the first stage of development the Sensorimotor Stage. The important effect on locomotion may best illustrate this concept.
Locomotor activity ensures the integration of a child's behavior in the environment and therefore plays a role in the child's communication and functioning. The three main components of locomotion include production of basic locomotor rhythm, maintenance of equilibrium, and adaptation of the activity to move toward the goal.
10 Studies have demonstrated significant deficits in the area of maintaining equilibrium, which could interfere with locomotion and interfere in the domain of communication.
11–13Locomotor control/activity, in conjunction with object-control skills, constitute the fundamental movement skills that emerge after the ability to walk, between the ages of 1 and 7 years. They are considered “fundamental,” as they span all ages and cultures and are considered the building-blocks of more complex, sport-specific skills.
14 Berkeley et al.
15 associated ASD with poor fundamental movement skills, as compared with peers without ASD. With respect to the locomotor skills, 80% of the children with ASD scored in the Poor or Very Poor range, and 53% scored poorly in terms of object-control skills.
On a more general level, links between sensory/motor functioning and cognition are well established across all groups, clinical and nonclinical. Indeed, higher-level brain functions have been shown to have a significant relation with sensory/motor functions.
1 Ties have been established between sensory/motor functioning and visual and motor imagery, iconic memory, temporal judgment, mental rotation, action concepts, higher-order cognitive functioning, and academic achievement, among other neurocognitive domains.
16–19 These findings, among others, support the idea that cognition and sensory/motor functioning are intimately linked,
20 and that sensory and motor systems should be considered salient markers of cortical and subcortical integrity.
21Differentiation Based on Motor Functioning: The Present Study
Autism and Asperger's remain largely differentiated on the basis of language and communication skills. Motor functioning, which is largely independent of language development, has never been explored to differentiate the two disorders.
22 Previous studies comparing the various domains have found motor differences between the autistic and pervasive developmental disorder (PDD), Not Otherwise Specified groups, further adding to our knowledge that children with ASDs differ with regard to their motor skills.
23,24 The literature has established that neurological patterns and discrepancies exist among these groups;
25–30 thus, differences in motor functioning may be anticipated, given their relationship with overall CNS integrity.
31 Nevertheless, this has not been undertaken previously. To address this void, we sought 1) to determine what, if any, differences exist between groups in motor functioning; and 2) to determine whether such discrepancies, if they exist, are capable of differentiating groups. This was done based on the concept that neurological discrepancies between the two could be elicited purely through motor assessment. To accomplish this, we felt the assessment must not only be comprehensive, but also standardized. The Dean-Woodcock Sensory-Motor Battery (DWSMB)
32 met both criteria because it was designed to standardize the administration and interpretation of sensory/motor functions and has demonstrated satisfactory reliability. The DWSMB so far has not been used to assess the subtle differences in motor functioning between AS and autism groups, which provided an additional novel endpoint of this study.
METHODS
Participants
The current sample consisted of 39 patients who were originally referred for neuropsychological evaluation to a large, Midwestern neurological practice. Diagnoses were made by both a board-certified neuropsychologist and neurologist after reviewing the results of medical records, interviews, and neurological exams. Of this original sample, 13 were excluded because of missing data, leaving 26 participants across the two groups combined. The first group included 16 patients diagnosed with autism (14 male and 2 female patients), ranging in age from 6 to 23 years (mean age: 12.1; standard deviation [SD]: 5.16), with a level of education ranging from 1st to 12th grade (mean: 5 years of education). Most participants were Caucasian (87.5%), followed by African American (12.5%).
The second group consisted of 10 participants diagnosed with Asperger's disorder (8 male and 2 female participants). The participants ranged in age from 11 to 31 years (mean age: 21.3; SD: 8.75). Level of education ranged from 6th grade to graduate degree (mean: 11.5 years of education). The ethnicity of the group was Caucasian (100%). For both groups, individuals with a comorbid neurological presentation were excluded, including history of head injury, seizures, cerebral palsy, etc. Although we sought to exclude individuals with comorbid psychiatric presentations, so as to offer further control for the study, this diminished the numbers to such a degree that adequate power was no longer maintained. Individuals with comorbid psychiatric presentations were included in the sample; these including depressive and anxiety spectrum disorders.
We acknowledge that although inclusion of individuals with comorbid psychiatric features may be seen as a confound or limitation of the study, in many ways it is also a strength, as it remains true to form with what is observed in this population in the real world. Confounding issues may arise in the fact that motor deficits may occur in the presence of these other psychiatric features (e.g., motor retardation in the presence of depression). However, distribution of these comorbid presentations was relatively equal across the two groups.
Instrumentation
Patients were administered the Dean-Woodcock Sensory-Motor Battery (DWSMB), which is a standardized and norm-referenced measure of cortical and subcortical sensory/motor functioning based on the Dean-Woodcock Neuropsychological Model.
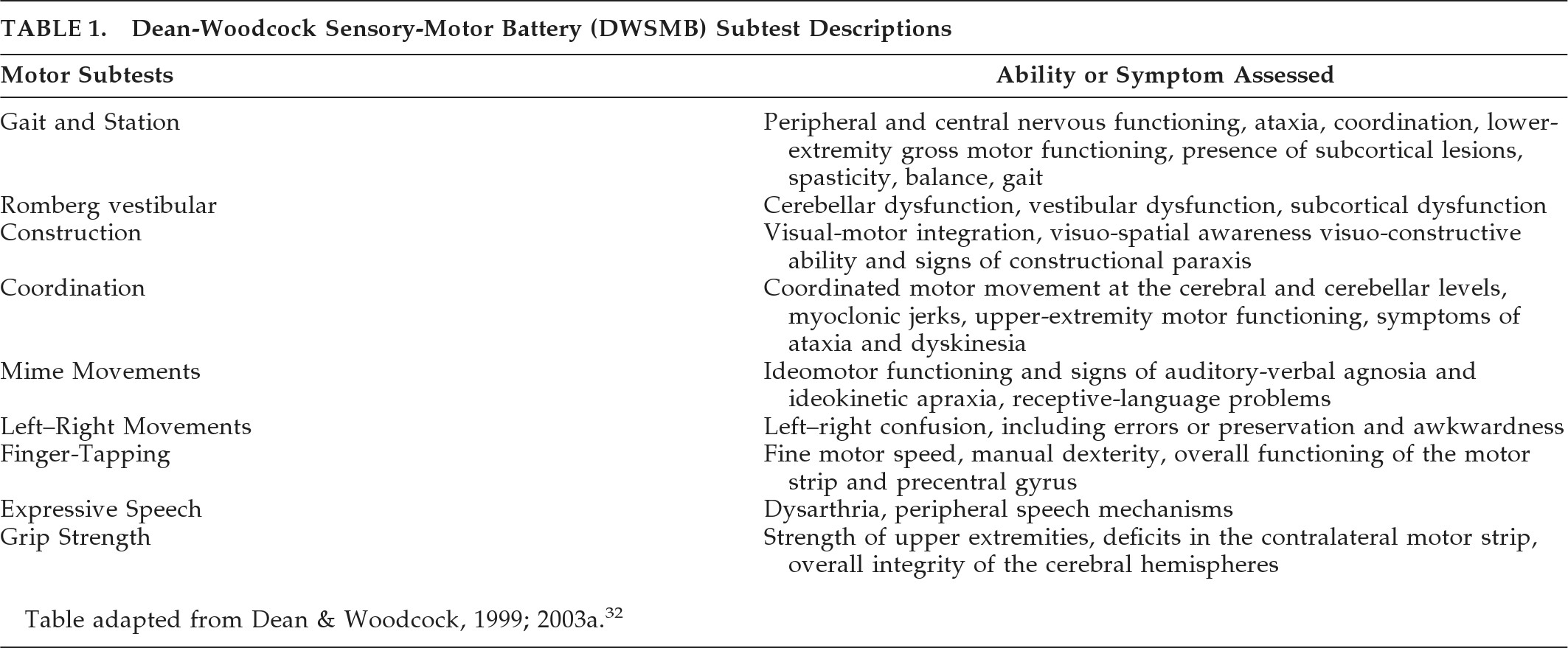
32 Six subtests were selected for comparison, including Gait and Station, Romberg, Cross-Drawing Construction, Clock-Drawing Construction, Finger-to-Nose Coordination, and Hand-to-Thigh Coordination. Finger-Tapping and Grip-Strength subtests were removed from the comparison because of missing data that dropped numbers below adequate power levels. A brief description of each subtest appears in
Table 1.
Procedure
Each participant was administered the DWSMB, using standardized administration procedures described in the DWSMB manual.
32 All examiners had been previously trained in the use of neuropsychological assessment instruments and were supervised by a board-certified clinical neuropsychologist during this administration. Raw scores were calculated and transformed into W scores. The W scale provides a common scale of interpretation and represents an individual's ability in a targeted area, taking into consideration the task's level of difficulty. The W scores were further converted to WD scores.
33 The WD scores are the difference between the individual W score obtained by a person on a subtest and the average W score for the reference group in the norming sample. Since the individual performance is compared with a norming sample, the WD scores partial out the possible effects of age and gender, and reflect the population ability-distribution. As such, using the WD scores allowed us to control for the effect of age and avoid the impact of the group age-differences on the final results of the analyses.
RESULTS
Multivariate Analysis of Variance
A multivariate analysis of variance (MANOVA) was performed, using the six aforementioned tests of the DWSMB
3 as dependent variables (i.e., Gait and Station, Romberg, Cross-Drawing Construction, Clock-Drawing Construction, Finger-to-Nose Coordination, and Hand-to-Thigh Coordination). Diagnostic grouping (i.e., autism or Asperger's) constituted the independent variable.
A MANOVA was conducted using SPSS for the analyses, with a hierarchical adjustment for nonorthogonality. The total sample (N=39) was reduced to 26 participants because of missing data from 13 participants. Participants with missing data were simply removed from the analyses, as this approach is considered a good alternative.
34 No univariate or multivariate within-cell outliers, at α=0.001, were found. Assumptions of normality, linearity, homogeneity of variance/covariance matrices, and multicollinearity were met. Also, the covariate of age was found to be reliable for covariance analysis.
Wilks' lambda criterion (λ) demonstrated that the combined dependent variables (DVs) were significantly related to group membership (i.e., diagnosis:
F [8, 13]=45.282; p<0.001), with very high association identified between the combined DVs and the main effect of diagnostic groupings (
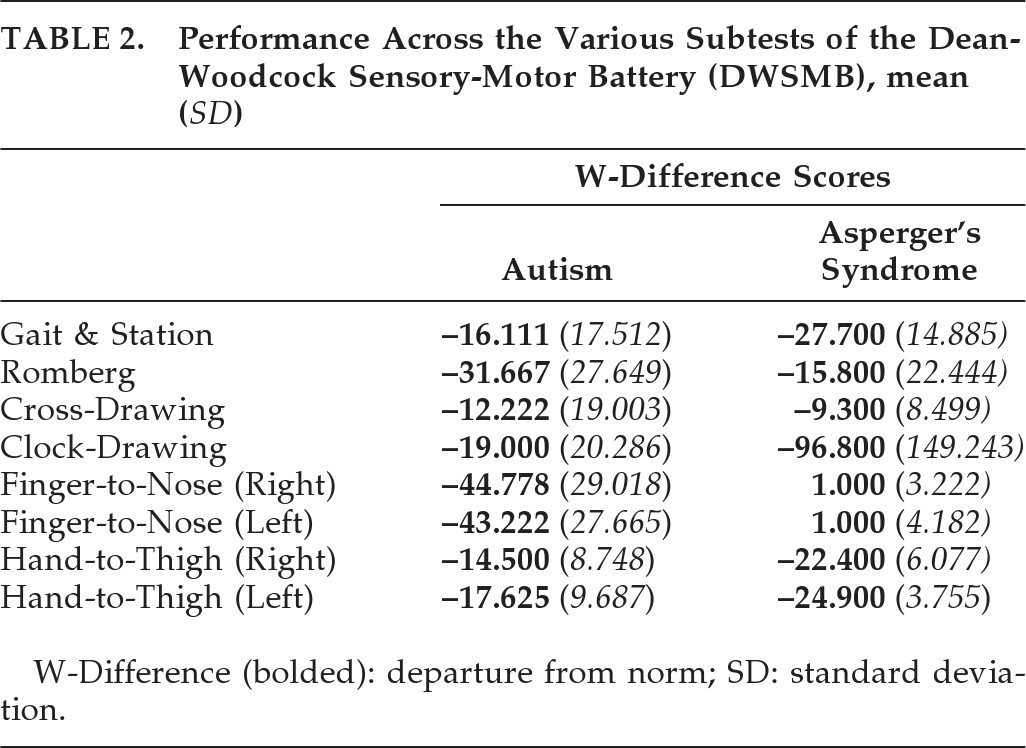
Table 2 displays performance across the various subtests of the DWSMB).
Discriminant Analysis
A direct discriminant-function analysis was performed, using the described motor subtests as predictors of membership in the two groups. Scores on the aforementioned tests of the DWSMB constituted the potential predictors. Group diagnoses (i.e., autism and Asperger's) served as dependent variables.
Of the original 39 participants, 13 were excluded from the analysis because of missing data.
34 No multivariate outliers, at α=0.001, were found. The remaining sample met assumptions of linearity, normality, multicollinearity or singularity, and homogeneity of variance/covariance matrices, thus alleviating threat to multivariate analysis. The “jackknife technique” (i.e., the “leave-one-out” classification technique) was selected as the validation method, which gives the most conservative estimation of predictive values. This permits assessment of how accurately these subtests predicted group membership. When including all variables, a significant discriminant function resulted, with a combined χ
2 [7]=70.876; p<0.001. The discriminative function was able to correctly classify 100% of cases as demonstrated by the jackknife technique.
Multiple independent-sample t-tests were run to offer further evaluation of group difference across each subtest. An α level of 0.006 was used for comparisons, after Bonferroni correction (0.05/8). Results demonstrated that significant differences emerged on two subtests, Finger-to-Nose Coordination with the right hand and Finger-to-Nose Coordination with the left hand, potentially suggesting discrepancies between groups in cerebellar functioning. However, differences approaching significance were also noted in Hand-to-Thigh Coordination, with both hands, as well as Clock-Construction. These were found to be nonsignificant as compared with the revised α obtained with the noted Bonferroni correction.
On the aforementioned subtests, individuals with Asperger's (M=1.00) performed significantly better than individuals with autism (M = –44.778) on Finger-to-Nose Coordination with the right hand: t [26] = –4.947; p<0.001. Similarly, individuals with Asperger's (M=1.00) performed significantly better than individuals with autism (M = –43.222) on Finger-to-Nose Coordination with the left hand: t [26] = –5.012; p<0.001. On those subtests approaching significance, the Asperger's group appeared to do worse than the autism group on both Hand-to-Thigh Coordination with the right hand (Asperger's M = –22.400; autism M = –14.500; t [24]=2.495; p=0.020) and the left hand (Asperger's M = –24.900; autism M = –17.625; t [26]=2.257; p=0.033). It is interesting to note that both groups present with impairment in this domain, versus the normal population, whereas only autism was associated with dysfunction, versus the normal population, on Finger-to-Nose Coordination with either hand. Similarly, in comparison with the normal population, both groups present with significant deficits in Gait and Station (Asperger's M = –27.700; autism M = –16.111), Romberg (Asperger's M = –15.800; autism M = –31.667), and Clock-Drawing (Asperger's M = –96.800; autism M = –19.000). Although a large discrepancy is noted between groups on Clock-Drawing, this difference remained nonsignificant (t [26]=2.208; p=0.036), appearing to be due to higher SDs and errors-of-the-mean exhibited by groups. Cross-Construction was not discrepant between groups and not very discrepant from the normative population (Asperger's M = –9.300; autism M = –12.222).
DISCUSSION
Autism and Asperger's are both characterized by varying degrees of social impairments; communication difficulties; and restricted, repetitive, and stereotyped patterns of behavior. The significant overlap in their clinical manifestations has previously led to the proposal of their representing manifestations on the same spectrum. More recently, greater consideration of their discrepancies has been of empirical interest even as DSM–V moves toward combining the groups under the Autism Spectrum Disorder heading. This has extended beyond differences in language functioning, which represents the most common factor used to differentiate the two.
In the case of the present study, motor functioning was of interest, as deficits within this domain have been described in association with both disorders. With growing evidence of discrepancies in the neurophysiology of these two disorders, and knowledge that sensory and motor functioning can often be a window into overall CNS integrity, it was hypothesized that differences would not only emerge, but they may be of such a nature that they would suggest usefulness of such data in differential diagnosis. By use of a multivariate analysis, followed by a post-hoc discriminant function, we found that only do the groups differ, but their profiles were of such a nature that a “leave-one-out” classification (i.e., “jackknife”) was able to successfully identify 100% of participants through actuarial means.
Because of small sample size, subtest-specific findings were diminished more than we estimate would be the case with an adequate N. Although the discriminant function was significant, as a whole, subtests tended to fall below our conservative cutoff of 0.05 as the determinant of significance. Multiple t-tests were thus undertaken, while using a Bonferroni correction to control for inflation of family-wise error rate. In doing so, significant differences were seen between groups when evaluating Finger-to-Nose on left and right hand, with impairment seen in the autism group. In fact, the Asperger group demonstrated performance within normal limits.
Representing a measure of cerebellar functioning, these positive findings are consistent with anatomical research reported to-date. Microscopic reviews have demonstrated loss of Purkinje cells within the cerebellums of individuals with autism,
35 yet abnormalities in the cerebellum have not been noted in association with Asperger's.
Although Finger-to-Nose Coordination was the only area of significant group differences, additional findings were likely limited by the low sample size. For example, on Hand-to-Thigh Coordination with both the left (p=0.033) and right (p=0.02) hand, subjects with Asperger's appeared to do worse. However, again, in light of the new alpha (i.e., α=0.006) these discrepancies were not significant. Whereas Finger-to-Nose is a test of placement, and reflects the function of lateral cerebellum functioning, the Hand-to-Thigh test reflects functioning of basal ganglion and corticospinal tracts along with lateral cerebellum.
36 Consequently, reductions in basal ganglion physiology and volumetric reductions in gray matter have shown to be present in Asperger's disorder on neuroimaging studies,
37 possibly explaining abnormal hand-to-thigh dyscoordinaton in Asperger's disorder, even though the above results were not in the significant range.
When evaluating group outcomes in comparison to standard clinical cutoffs established by the normative sample, both autism and Asperger's were associated with deficits in Gait-and-Station and the Romberg test. Although no differences existed between the experimental groups, these findings reiterate previous research demonstrating broader motor deficits in association with both manifestations and specific difficulties in proprioception and postural anticipation and multi-joint coordination during locomotion.
38–40 Also, deficits in clock-drawing within both groups could be explained by poor fine motor/graphomotor control, which is consistent with previously-discussed findings.
23Inherent in all studies are limitations. In the present study, our primary limitation was low sample size. As a result of this limitation, replication of this study with larger groups is warranted, as there is currently a stronger possibility of Type II error in those subtests that did not reach significance that may not have attained it because of the low numbers in the sample. Also, there was significant variability in the ages of participants. By using WD (W-difference) scores, age differences were controlled for before analysis; yet, future studies should attempt to use groups with a greater degree of age-matching. In our case, this was necessary to obtain adequate numbers, which were still low.
CONCLUSION
This was one of the first studies demonstrating that motor differences can be used to accurately classify autism and Asperger's syndrome, even though motor deficits have been associated with both groups. Interestingly, as we move toward DSM-V classification, and merging the two disorders into one spectrum, emergence of findings such as these suggest inherent differences between the two groups that can, in many ways, be equated to neuroanatomical differences. The question that must then be asked is whether such a merging is appropriate. One may argue that a single spectrum disorder is a better reflection of the state of knowledge about clinical presentation (DSM-V proposed changes). Within such a mind-set, one may argue that the differences demonstrated in this study merely reflect outcomes of a sub-spectrum feature of the single disorder. In comparison, one may also argue that differences such as those identified in this study further suggest true neurological differences between the two disorders, which would support a position of keeping Asperger's and autism separate in their classification.
Our findings beg the question that even if two presentations demonstrate significant clinical overlap, if there is empirically-based evidence that shows areas of consistent discrepancy, is that not enough to call them separate? At the very least, this study suggests that significant differences exist between the two disorders, even though they may be reclassified as part of a single spectrum and on opposite poles. Furthermore, there is no mention in the DSM-IV-TR regarding the use of these motor differences in classification. DSM-V has also not included or mentioned motor aspects of the disorder as part of their severity scales, although there is ample literature suggesting that this as a common consistent feature. Although sensory/motor assessment remains common in neurology, psychiatry, and related fields, in presentations such as autism and Asperger's, there is less concentration on these data. The present study reinforces the concept that these factors can be reliable measures of aspects of CNS integrity. With further research and more study, this information could be incorporated into the classification system of these presentations, whether or not they are reclassified as part of the same spectrum but on different ends of the pole.