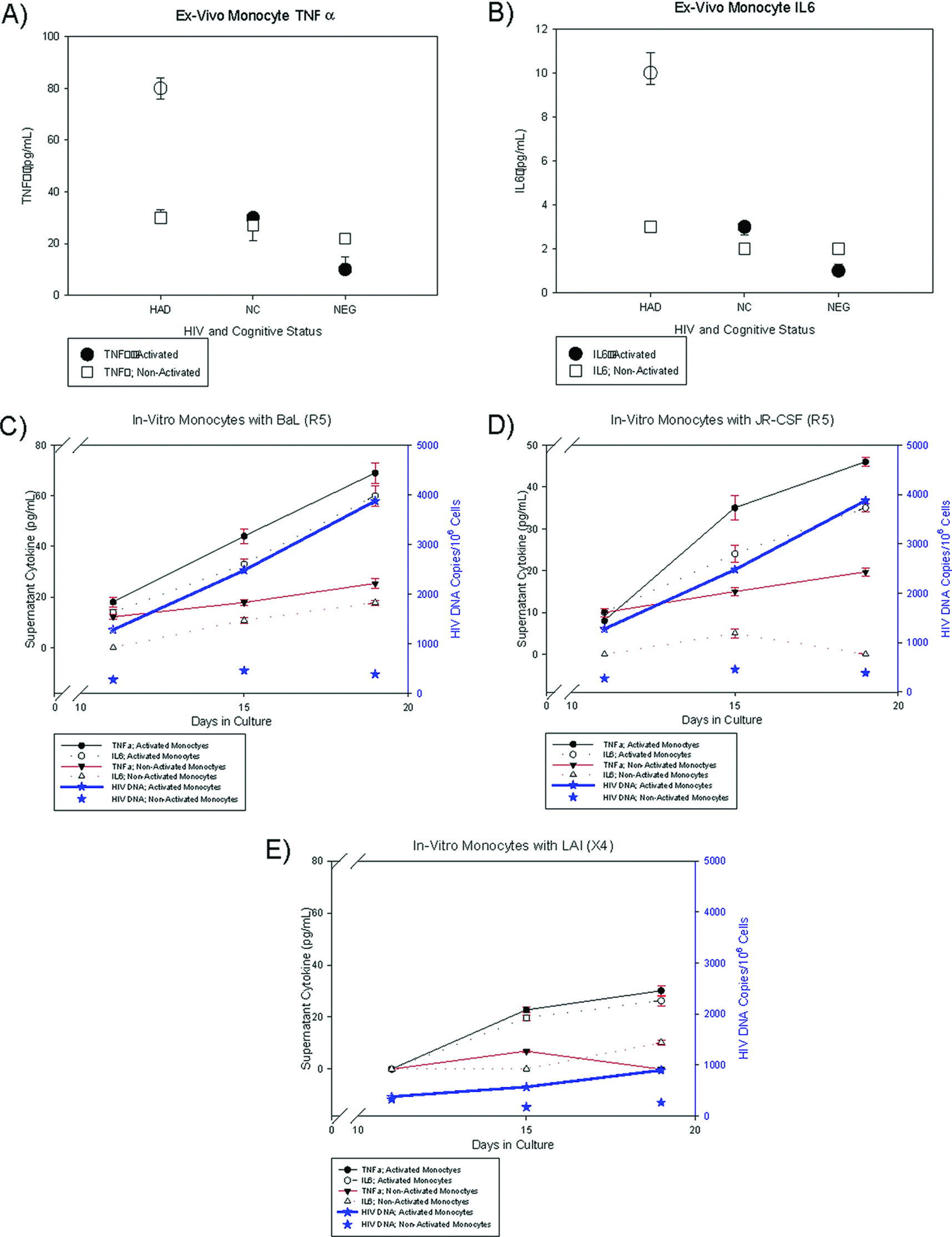
Ex-vivo and in-vitro experiments were designed to characterize cytokine profiles of cellular subsets, which included PBMC and activated monocytes with HIV DNA.
Ex-vivo experiments were set up with specimens from three groups of patients whose PBMC were previously analyzed for HIV DNA copies as well as from HIV-negative control volunteers: 1) patients with high (log HIV DNA>2) PBMC HIV DNA copies; 2) patients with undetectable PBMC HIV DNA copies; and 3) HIV-seronegative control volunteers.
9 Activated and non-activated monocytes (CD14
+16
+ and CD14
+16
−) from patients in each group were isolated as noted above and plated at a density of 3 × 10
6 cells per cm
2 and maintained at 37°C with 5% CO
2. Cells were washed 24 hours after plating to purify for adherent monocytes and maintained for 10 days in media (25% RPMI Media; Sigma, Inc., St. Louis, MO; Isocove's modified Dulvecco's medium; Irvine Scientific, Santa Ana, CA; 4 mM
L-glutamine, Sigma, Inc.; 100 units/ml penicillin; 100 μg/ml streptomycin; Sigma, Inc.; 50 U/ml MCSF, Sigma, Inc.; 1 ng/ml IL3; 10% human serum; Sigma, Inc.). Equal numbers of cells were plated (96 or 48 well-plates; 9 × 10
5 cells/well and 3 × 10
6 cells/well) depending on the number of cells recovered. After 7 days, the media components were changed (excluding MCSF and IL3) and continued in culture for 3 more days. On Day 10, supernatants were obtained from CD16
+ and CD16
− monocyte cultures analyzed for inflammatory cytokines. Quantikine ELISA kits (R&D Systems, Inc.; Minneapolis, MN) were used according to manufacturer's instructions to assay for IL6, IL8, IL10, TNFα, sFAS, sTNFRI, sTNFRII, and MCP1. For the in-vitro experiments, monocytes were separated from commercially-available PBMC (AllCells; Emeryville, CA) using the EasySep Human CD14 Positive Selection Kit (Stem Cell Technologies; Vancouver, BC, Canada). After separation, approximately 24 × 10
6 monocytes were seeded in a 10-cm HydroCell Surface plate (Nunc; Rochester, NY) in 12.5 ml of Isocove's Modified DMEM (Mediatech; Manassas, VA) supplemented with 10% fetal bovine serum (Sigma-Aldrich; St. Louis, MO), 1% penicillin/streptomycin, 1% sodium pyruvate, and 1%
L-glutamine. After 2 days in culture, approximately 12 × 10
6 monocytes were exposed to Human M-CSF (Miltenyi Biotec; Auburn, CA) at a concentration of 50 U/ml in a 6-cm Hydrocell surface plate in 8.5 ml of Isocove's Modified DMEM, as previously outlined, in order to yield activated (CD14
+16
+) monocytes, which were previously confirmed by flow cytometry on an aliquot of cells from the plate. After a 1-day activation period, the activated monocytes were washed and resuspended in Isocove's Modified DMEM without M-CSF. The other 12 × 10
6 monocytes were continued in suspension without the addition of M-CSF to yield non-activated (CD14
+16
−) monocytes. Each culture (in triplicate) of activated and non-activated monocytes was exposed to different strains of HIV consisting of R5– (BaL, JR–CSF), X4– (MN, LAI), and dual-tropic (p89.6) HIV with a multiplicity of infection of 0.05 in Isocove's Modified DMEM, supplemented with 2% fetal bovine serum. After an 8-hour exposure, respective viruses were washed from the cell suspensions, and the remaining viable cells were maintained in culture with Isocove's Modified DMEM, supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin for a 1-day period. After recovery in DMEM supplemented with 20% FBS, both activated and non-activated monocyte cell cultures were maintained in culture for a total of 21 days post-infection with Isocove's Modified DMEM, supplemented with 10% fetal bovine serum and additional supplements as previously outlined. HIV p24 levels were assayed (ExpressBio; Westbury, NY) daily to determine optimum HIV-infected days to begin measuring cytokines. Supernatants were collected on Days 11, 15, and 19, with residual cells lysed for HIV DNA assay, had cytokines (TNFα, IL6, IL8, GMCSF, and IL1β) measured by Luminex analysis, using a 5-plex Invitrogen plate as per manufacture's guidelines. The supernatant volumes were limited; therefore, the cytokines were measured by Luminex instead of using ELISA. Supernatant cytokines from infected cell-lines were analyzed in triplicate and normalized to cytokines measured from concordant cell line cultures set up in parallel without virus.