Brain-derived neurotrophic factor (BDNF) regulates neuronal function by promoting survival of neurons and enhancing synaptic plasticity via TrkB receptors.
2 On the basis of the neurodevelopmental hypothesis, the BDNF gene is a good candidate locus for schizophrenia. Several reports have found alterations of BDNF protein levels in the cortical area and hippocampus of schizophrenic patients.
3 Moreover, the first episode of schizophrenia is accompanied by a significant reduction in BDNF serum level.
4The val66met (rs6265) is a functional polymorphism in the coding sequence of the BDNF gene. It has been shown that the met allele affects intracellular trafficking and activity-dependent secretion of BDNF.
5,6 There are still some discrepancies among the results of association studies between schizophrenia and BDNF val66met polymorphism in Caucasian populations.
7–9 Despite the fact that schizophrenia is a heterogeneous mental disease characterized by a number of symptoms, surprisingly, there are no papers focusing on a single subtype of schizophrenia.
The present study concentrates on the paranoid subtype of schizophrenia. First, we aimed to determine whether val66met polymorphism of the BDNF gene is a risk factor for the development of this subtype schizophrenia in a Caucasian population. Second, we examined whether val66met polymorphism is associated with age at onset and psychopathology of paranoid schizophrenia. Also, we assessed the associations taking into account gender of patients.
Methods
Genomic DNA was isolated from peripheral blood samples by phenol-chloroform extraction. The val66met polymorphism of the BDNF gene was determined by the use of the polymerase chain reaction-restriction fragment-length polymorphism (PCR-RFLP) method. An amplification of the region containing the studied single nucleotide polymorphism (SNP) was achieved using the nested PCR strategy. Amplification reactions in the first- and second-step PCR were performed in 25 µl of reaction mixture containing 0.25 mM dNTP, 2.5 µl DreamTaq buffer 10×, 1.25U DreamTaqDNA polymerase (Feremetas), and 0.4 mM specific primers. In the first-step PCR, 300 ng DNA and external primers were used. Cycling parameters were the following: 95°C for 3 min.; 40 cycles at 95°C for 45 sec., 53°C for 35 sec., 72°C for 45 sec., and final elongation at 72°C for 10 min. A 2-µl aliquot of the amplified PCR product was transferred to the new mixture containing internal primers. Cycling parameters of the second-step PCR were: 95°C for 2 min., 35 cycles at 95°C for 35 sec., 51°C for 30 sec., 72°C for 25 sec., and final elongation at 72°C for 10 min. The PCR primers used for amplifications were the following: external [sense 5′-CCCCATGAAAGAAGCAAACA-3′; antisense 5′-TTTGTCTGCTGCCGTTACC-3′; amplicon length 403 bp]; internal (sense 5′-GGACTCTGGAGAGCGTGAATG-3′, antisense 5′-GAGAAGAGGAGGCTCCAAAGG-3′; amplicon length 200 bp]. Internal primers were designed for reference sequence AC087446, Version AC087446.13 (NCBI base). Amplification was performed using G-Storm GS1 thermal cycler (Gene Technologies, LTD, Essex, UK). PCR products were analyzed by agarose electrophoresis in 2% agarose gel stained with ethidium bromide.
RFLP Analysis
The BDNF val66met genotypes were determined with RFLP analysis. The PCR product was digested with NlaIII (Fermentas). Products of digestion were separated and analyzed in 3% agarose gel stained with ethidium bromide. For the met allele, 123-bp and 77-bp fragments, and, for the val allele, 200-bp (undigested) fragment were observed.
Statistical Analysis
Descriptive variables are presented as mean (standard deviation [SD]. The distribution of alleles and genotypes frequencies between control and patient groups were compared either by the χ
2 test, the maximum-likelihood χ
2 test, or Fisher’s exact test. Two-way ANOVA with interaction was used for comparisons of PANSS subscales and age at onset of schizophrenia between groups. The Student
t-test was used to compare PANSS items between patients with different genotypes and age of patients and controls. The variables’ distributions were evaluated by the Shapiro-Wilk test. Homogeneity of variance was assessed by the Hartley test. Statistical calculations were performed with Statistica software, Version 8.0 (
www.statsoft.com). All p values were two-tailed, and statistical significance was established at p <0.05.
Discussion
In the present study, we analyzed the val66met polymorphism of the BDNF gene in a Caucasian (Polish) population. Our research was focused on a homogeneous patient group, suffering from the paranoid schizophrenia subtype. Schizophrenia patients with coexisting depressive episodes and schizoaffective disorders were excluded. Our case–control study has not shown an association between val66met polymorphism and paranoid schizophrenia occurrence in either men or women. Numerous case–control studies and metaanalyses conducted in Asian populations have also failed to find an association between BDNF val66met polymorphism and schizophrenia.
9,11,12 On the other hand, studies in Caucasians are still ambiguous. In agreement with our results, most of the case–control studies have not revealed an association between the val66met polymorphism and incidence of schizophrenia.
7,13–15 However, Neves-Pereira et al.
8 pointed to a schizophrenia association in the val allele. Discrepancies in the results of meta-analyses are observed, as well. Jönsson et al.
14 have revealed an association of homozygous states; Gratacòs et al.
16 have found that the met/met genotype is a risk factor for development of schizophrenia, but Xu et al.
9 have not found any association.
Some previous studies pointed at a potential connection between the BDNF gene and schizophrenia symptoms. BDNF is an important trophic factor for dopaminergic,
17 GABAergic,
3,18 serotoninergic, cholinergic, or noradrenergic neurons,
19 which are known to be dysregulated in schizophrenia. Alterations of GABA or dopamine neurotransmission may contribute to psychopathological symptoms of schizophrenia.
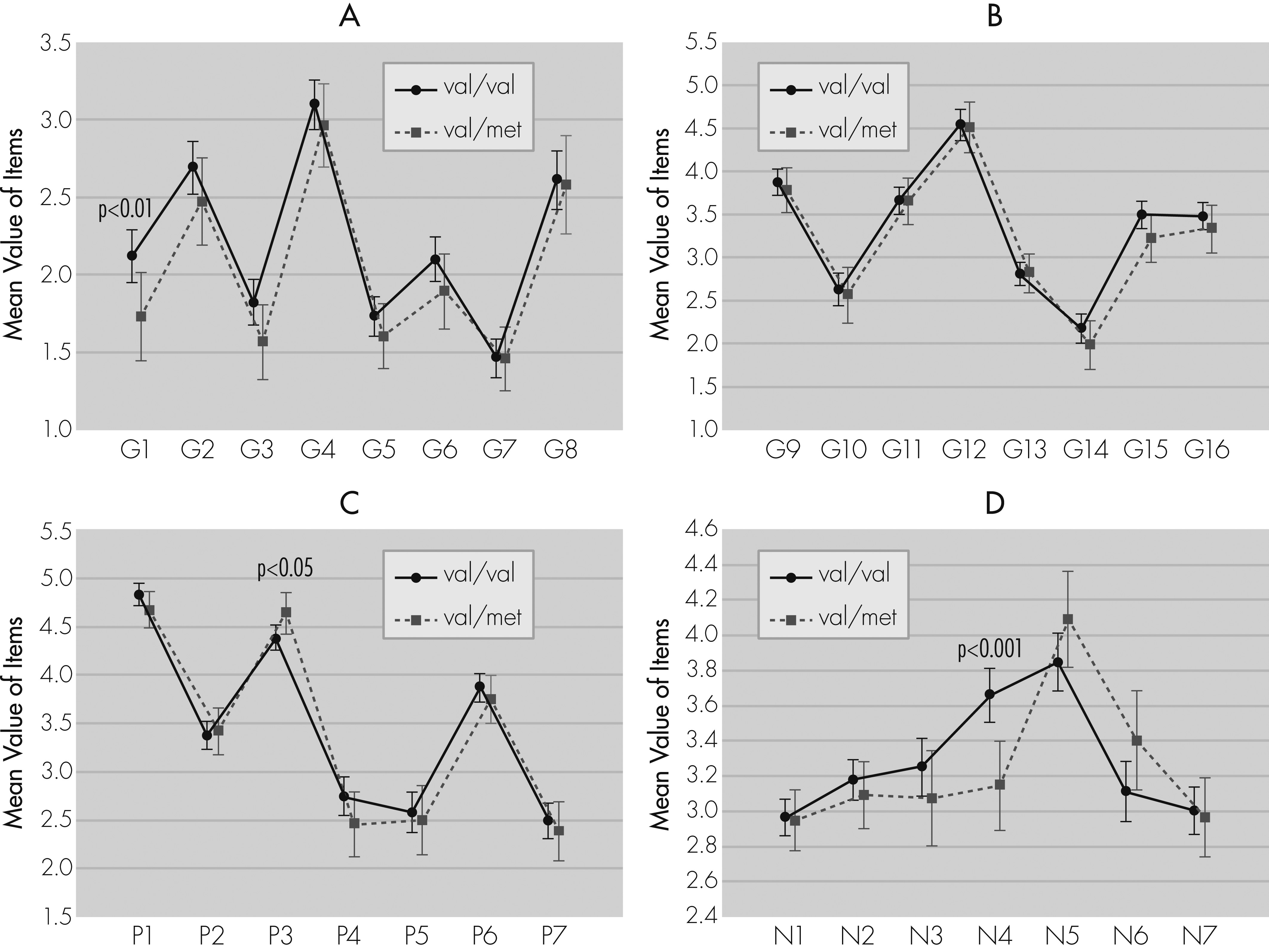
20,21 The PANSS scale is the most widely used to measure severity of symptoms in schizophrenia. The current research has revealed an association between BDNF val66met polymorphism and the PANSS scale. It has been shown that male patients with the val/val genotype have a worse clinical course of disease. Our results are in line with the study performed on a large group of Russians, showing a severe clinical course of schizophrenia in men with the val/val genotype.
13 Similar to our findings, Chang et al.
22 have found higher mean scores for the G scale and Total PANSS scales in patients with the val/val genotype, as compared with patients with other genotypes, although, in contrast to our results, they have also observed higher mean scores for the N scale and no differences on the P scale between genotypes. Spalletta et al.
23 have not shown differences between genotypes and the PANSS scale in schizophrenia outpatients. However, an analysis of the Modified Overt Aggression Scale (MOAS) revealed an association between the met allele and aggressive behavior, especially verbal aggression. It is noteworthy that the family-based study showed a preferential transmission of val allele from heterozygous healthy parents to affected by schizophrenia offspring.
24Our analysis of the association between BDNF genotype and time of the first episode of schizophrenia has revealed that men with the val/met genotype had an earlier age at onset then women with the same genotype. It is in agreement with previous findings, which also showed an earlier age at onset for met allele carriers (those who display met/met or met/val genotypes).
25,26 On the other hand, some studies have not confirmed such differences.
7,27Discrepancies between our results and those of other studies can result from the fact that we assessed only patients with the paranoid subtype of schizophrenia. Studies discussed above encompassed different subtypes of schizophrenia,
22–25 and included patients suffering from schizoaffective disorder.
27 It is possible that participation of the BDNF gene may vary in distinct subtypes. It is worth mentioning that serum BDNF protein level is different in the course of various subtypes of schizophrenia.
28 It is also possible that single-nucleotide polymorphisms (SNPs) in the BDNF gene affect only some of symptoms of schizophrenia. Schumacher et al.
15 revealed differences in the frequency of haplotypes for the set of SNPs in the BDNF gene (also including the val66met SNP) between schizophrenic patients with and without coexisting depressive episodes. Interestingly, haplotypes overrepresented in schizophrenic patients with coexisting episodes of depression were also more frequent in depressed patients.
15 Golimbet et al
13 have found that the val/val genotype was more frequent in men with continuous schizophrenia than in men with episodic schizophrenia.
We also conducted an evaluation of the val66met polymorphism in relation to PANSS single-item scores. As far as we know, this is the first work in which such analysis has been performed. We have noted that patients with the val/val genotype scored higher on the following items: passive/apathetic social withdrawal, somatic concern, guilt feelings, and preoccupation. However, hallucinatory behavior was connected with the val/met genotype.
Some authors have suggested the possibility that BDNF participates in the pathophysiology of suicidal behavior. Both the mRNA and protein levels of BDNF and its receptor TrkB have been significantly decreased in the brain of suicide subjects.
29 The alterations in epigenetic modifications of the BDNF gene have been revealed in suicidal behavior. DNA methylation levels at the BDNF promoter were increased in suicide subjects. Moreover, this hypermethylation was connected with a lower amount of BDNF transcript IV.
30 Unfortunately, we have not found any association between the BDNF val66met genotypes and suicide attempts; this may be due to the small number of subjects. Other researchers have shown that the val/met and the met/met genotypes could be a risk factor for a suicide in women, but not in men. Moreover, these genotypes were frequently found in female suicide victims who used violent methods of suicide and in victims exposed to childhood trauma.
31 The val/met and met/met variants were also found to increase the risk of suicidal behavior among depressed patients.
32Summarizing, in this report we have conducted an analysis of BDNF val66met functional polymorphism in a homogeneous group of patients with the paranoid subtype of schizophrenia and without coexisting depressive episodes or schizoaffective disorders. There was no association between the BDNF val66met polymorphism and schizophrenia occurrence, but an association was found with regard to the clinical course of disease. In men, but not in women, the val/met genotype was connected with earlier age at onset, whereas val/val genotype was associated with more severe symptoms, particularly general symptoms (PANSS-G scale). However, PANSS single-item score analysis has shown that hallucinatory behavior was more intense among the val/met patients. The main limitation of our study is its relatively small sample size. Therefore, larger studies should be performed to confirm these findings.