Delirium is an acute or subacute disturbance of consciousness, cognition, and attention that usually fluctuates over time.
1 It is a common disorder in the intensive care unit (ICU), with a reported incidence up to 80% during the ICU stay.
2,3 Delirium is associated with higher mortality, longer hospital stay, more long-term cognitive impairment, and increased costs.
4–8 Despite its frequency and impact, recognition of delirium by ICU physicians is poor (sensitivity: 29%).
9 Therefore, the Society of Critical Care Medicine (SCCM) and the American Psychiatric Association (APA) recommend daily monitoring of delirium in ICU patients to improve early diagnosis and treatment.
10,11 Various delirium assessment tools have been developed for use by nonpsychiatric personnel. Of these tools, the Confusion Assessment Method for the ICU (CAM-ICU) showed highest sensitivity in a research setting, ranging from 64% to 97%.
9,12 However, in routine, daily practice the sensitivity of the CAM-ICU appeared to be much lower (47%).
13 Sensitivity for hypoactive delirium was particularly poor (31%), which is the subtype most difficult to recognize by ICU physicians.
9,13 In this multicenter study, 282 patients were assessed by teams of three delirium experts, including psychiatrists, geriatricians, and neurologists, who based their assessment on cognitive examination, inspection of medical files, and established criteria
1 for delirium.
13 The low sensitivity of the CAM-ICU in daily practice hampers early detection of delirium. Other drawbacks of the CAM-ICU are that it cannot quantify delirium severity and that it assesses delirium at a certain moment in time,
12 whereas delirium may fluctuate considerably over the day.
1 These factors impede recognition and thereby delay treatment. Delayed treatment of delirium was found to be related to mortality.
14 Therefore, an objective detection tool for continuous monitoring of delirium is needed.
Another approach to detecting delirium is by monitoring physiological alterations. Delirium is a manifestation of encephalopathy; however, sometimes these terms are used interchangably. For decades, it has been known that during delirium, electroencephalography (EEG) shows generalized slowing of background activity.
15 Recently, continuous EEG monitoring with automatic processing has become technically feasible.
16 An example is the assessment of depth of anesthesia, which can be monitored with a limited number of EEG electrodes.
17 In ICU patients, continuous EEG monitoring is increasingly used to detect nonconvulsive epileptic seizures.
18 EEG-based encephalopathy monitoring with a limited number of electrodes and automatic processing, is therefore feasible. It is, however, currently unclear which EEG characteristic is particularly altered in delirium, and which EEG lead is most informative. In this systematic review, we aimed to explore which parameter and which EEG lead show the largest difference between patients with and without delirium.
Methods
Search Strategy
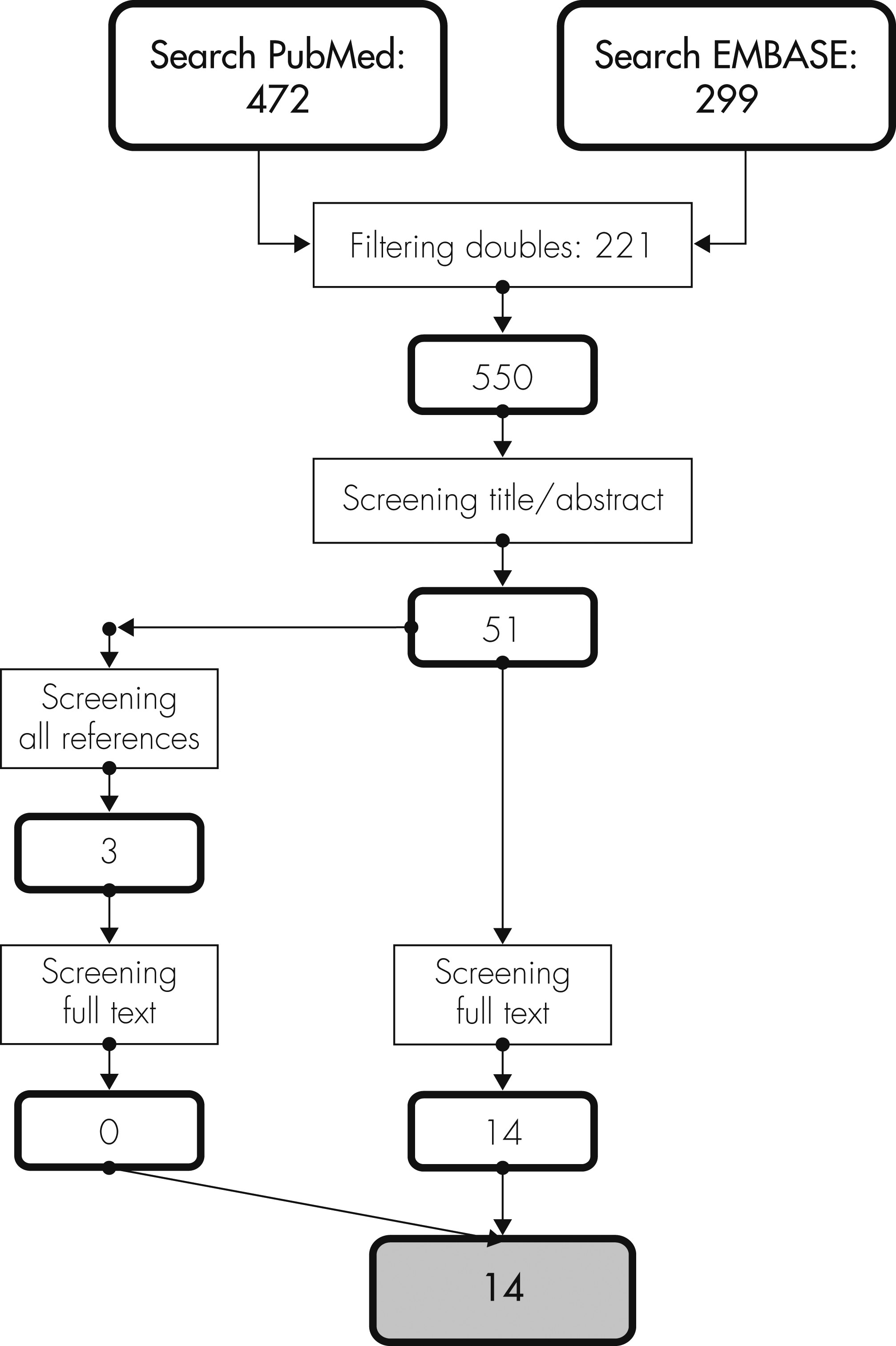
We systematically searched literature on delirium and EEG on November 25th, 2011, using the databases of EMBASE (January 1980 to November 2011) and PubMed (January 1966 to November 2011). Synonyms for delirium and corresponding Mesh terms were combined with search terms for EEG synonyms, and Mesh terms as can be seen in the online data supplement. Resulting English, French, German, and Dutch scientific articles on humans were imported into Reference Manager (Reference Manager Professional Edition, Version 11; Thomson ISI ResearchSoft). The reference lists of all full-text articles were also screened, and relevant publications were imported into the database.
Selection of Studies
The results of the literature search were evaluated independently by two authors (AWvdK, RJvdW). Based on title and abstract, we excluded case reports, animal studies, reviews, letters, and studies in children. Furthermore, articles that described the EEG of delirium subjects only in a descriptive way were excluded. Moreover, when the statistical significance of the difference in quantitative EEG characteristics between delirium and non-delirium subjects was not tested; articles were also excluded unless the statistical significance could be determined on the basis of the published data. All eligible studies were retrieved in full text. The reference lists of all full-text articles were also screened, following the same criteria used for screening titles and abstracts. In case of disagreement between the two authors, references were evaluated by three authors (AWvdK, RJvdW, AJCS) to reach consensus on inclusion for this systematic review.
Data Extraction
The selected full-text articles were closely reviewed, and the first author, title, and year of publication were noted. Furthermore, the following features were tabulated: size and characteristics of the study population, methods of diagnosing delirium, time between delirium diagnosis and EEG recording, EEG derivations for quantitative EEG analysis, use of artifact-free or raw data for quantitative EEG analysis, length of EEG period used for quantitative analysis, studied EEG parameters, and differences between patients with and without delirium with regard to these EEG parameters that reached statistical significance (p <0.05). Background information concerning quantitative EEG parameters can be found in the online data supplement.
For those articles in which the statistical significance had to be determined on the basis of the published data, a Student’s t-test was used to compare delirium subjects to non-delirium subjects. When data were not normally distributed, a Mann-Whitney U test was used.
Discussion
To develop EEG-based delirium monitoring with a limited number of electrodes and automatic processing, it is essential to know which parameter and which EEG lead show the largest difference between delirium and non-delirium patients. We evaluated various EEG parameters. Of these, the relative power of the theta frequency band was most frequently studied, and, without exception, different between delirium and non-delirium subjects. Investigations on quantitative EEG and delirium were mostly conducted in elderly patients and often used posterior EEG derivations only.
In order to perform continuous monitoring of delirium, a minimal number of electrodes are needed to increase feasibility and minimize the burden for the patient. Several publications mention that EEG changes in delirium are most prominent in the posterior regions, although this was not supported by original studies
22,23,25 Furthermore, none of the 14 included studies determined at which EEG derivation the EEG changes in delirium were strongest.
All 14 included studies used rather short periods of artifact-free EEG data to determine EEG characteristics. Fluctuation of EEG characteristics during these periods was not considered, although fluctuation of EEG characteristics over several days was studied by a number of authors.
19,24–27 Therefore, the exact relationship between the clinical fluctuation of delirium and the EEG characteristics of delirium is still uncertain.
Because of these fluctuations,
1 it is important to diagnose delirium soon before or after the EEG recording. Therefore, a long timeframe between delirium diagnosis and recording of EEG is a serious shortcoming. In only three studies were the delirium diagnosis and EEG recording performed at almost the same time.
20,27,31To allow continuous EEG monitoring of delirium, it is necessary to distinguish delirium from other conditions. Slowing of background activity occurs not only in delirium, but also in dementia and sleep.
22,32 One article on dementia in intensive care unit (ICU) patients reported the prevalence of dementia to be 17% in patients older than 65 years.
33 However, studies on the prevalence of dementia in the ICU are limited. In a recent multicenter study including 2,116 ICU patients, not more than 1.7% of the patients were reported to suffer from dementia.
34 Patients with dementia are usually treated substantially less aggressively than patients without dementia and are therefore less likely to be admitted to the ICU.
35 Therefore, we suspect that, in a general ICU population, the prevalence is much lower than 17%. However, even if a substantial proportion of ICU patients were suffering from dementia, delirium can be distinguished from dementia by recording of an active (eyes constantly open) EEG. The relative power in the delta and upper half of the alpha frequency band in an active EEG differ significantly between delirium and dementia patients.
22Nevertheless, deep sleep is also characterized by slow-wave activity.
32 The 14 studies included in this review all measured EEG during waking or light sedation and did not compare EEG characteristics of delirium with EEG characteristics of physiological sleep. EEG in sleep, however, has certain characteristics such as k-complexes and sleep-spindles,
32 which could be used to distinguish sleep from delirium, but this has not been investigated yet.
Despite an extensive literature search, only 14 articles could be found on quantitative EEG in delirium patients. Delirium is a frequent and serious problem in the ICU, but almost half of the quantitative EEG studies in delirium focus on elderly non-ICU subjects. Furthermore, we could not consider medication use in the different studies, although medication as haloperidol, which is often used during delirium,
10 may influence the EEG. Several studies reported EEG changes in healthy volunteers during haloperidol use when compared with placebo. These changes were, however, not uniform.
36–38 Therefore, the exact effect of haloperidol at a given dose on the EEG is unknown.
A future study should determine whether the relative power of the theta frequency band is indeed the most affected EEG characteristic. Furthermore, it is important to determine which EEG deviation is most sensitive for EEG changes related to delirium. If the most informative electrode deviations are known, one can limit the number of electrodes needed for continuous monitoring. Next, we should focus on continuous monitoring, with a limited number of electrodes, and determine the sensitivity of EEG-based delirium monitoring in a random sample of ICU patients over time. Given the feasibility of continuous EEG monitoring for epilepsy in ICU patients, this seems to be a promising approach to overcoming the underdiagnosis of current delirium screening tools.
Although some aspects of EEG in relation to delirium still have to be addressed, continuous EEG monitoring offers opportunities. Delirium can be adequately diagnosed in various populations by use of EEG. The relative power of the theta frequency band is the most promising parameter to study in a future prospective investigation in ICU patients.