Multiple linkage and association studies proved that many genes participate in the development of schizophrenia. Metaanalysis conducted by Allen et al.
2 confirmed the association of 24 single-nucleotide polymorphisms (SNPs) in 16 genes (
COMT,
DRD1,
IL1B) with schizophrenia. Genes encoding cytokines seem to be good candidate genes for schizophrenia. Cytokines have important functions in the central nervous system (CNS). They function as essential mediators of cross-talk between the brain and immune system and play a key role in neuroinflammatory processes. Many of the cytokines are normally produced in the healthy brain, where they play critical roles in such aspects of neurodevelopment as neurogenesis, migration, differentiation, and synapse-formation.
3,4 They could also interact with neurons, influencing neurotransmission. For example, by binding to specific receptors on the neuron’s surface, cytokines may modulate the secretory activity of these cells in relation to catecholamines or neuropeptides.
5 In previously published reports, concentration of various cytokines were found to be increased or decreased in patients with schizophrenia.
6 What is more, concentration of cytokines in blood serum of schizophrenic patients may vary depending on whether the patient is in active or resting phase of the disease.
7 Some authors suggest that schizophrenia may be associated with alterations in the Th1 (Il-2, IFN-γ)/Th2 (IL-6, IL-10) cytokine ratios (possibly induced by a viral infection), with a shift toward the Th2 system.
1,8,9IL-6 has a close functional relationship with IL-2 and TNF-α.
13 This cytokine is produced by a variety of cell types (e.g., macrophages, monocytes, fibroblasts) and has a pleiotropic activity in various tissues. IL-6 can exhibit both pro- and anti-inflammatory properties, as demonstrated in IL-6 gene knock-out mice.
14 Several studies have found the connection between increased serum concentration of IL-6 and immune abnormalities in schizophrenia.
15 IL-6 is widely distributed in the brain, especially in the hippocampus and hypothalamus, and it is strongly connected with the production of neurotransmitters. IL-6 can stimulate neurons in-vitro to secrete dopamine and probably also other catecholamines.
16 The dopamine hypothesis of altered dopaminergic neurotransmission is one of the most popular biochemical explanation for schizophrenia development. It is postulated that an imbalance in the redox-state of the brain may be part of the underlying pathophysiology of schizophrenia by alterations in fast-spiking interneurons. Inflammatory mediators, such as IL-6, can tip the redox balance into a pro-oxidant state.
17 Moreover, high concentration of IL-6 is postulated to be associated with the duration of the disease and resistance to treatment.
18TNFα is a proinflammatory cytokine produced mainly by immune cells (i.e., macrophages and active T and B lymphocytes), but also, in the CNS, by neurons, astroglia, and microglia. This cytokine plays a central role in the immune system and, in the CNS, regulates growth and differentiation of nerve cells, modulates serotonergic neurotransmission, and regulates synaptic scaling and apoptosis.
19 Gene-encoding TNFα is located within the HLA region on chromosome 6p21.3, which has been reported to be associated with susceptibility to schizophrenia.
20 It was shown that concentration of TNFα in whole blood from schizophrenic patients is significantly higher than in the blood of healthy individuals.
21 However, Baker et al.
22 did not reveal any differences in TNFα, or in IL-1β, IL-6, and sIL-2R serum concentration between schizophrenic patients and controls.
Discussion
Schizophrenia is a multifactorial disease, with contributions from multiple susceptibility genes, epigenetic, and environmental factors. Although the exact cause of schizophrenia remains unknown, the possible role of the immune response system in the pathogenesis of schizophrenia has been indicated.
1Currently, we attempted to establish an association between the polymorphisms in the promoter regions of IL-2 (−330G/T), IL-6 (−174G/C), and TNFα (−308G/A) genes and paranoid schizophrenia in a Polish population. To our knowledge, this is the first study investigating the combined impact of IL-2, IL-6, and TNFα gene polymorphisms on susceptibility to paranoid schizophrenia. Because of the fact that particular schizophrenia subtypes are characterized by different clinical pictures, it is reasonable to perform genetic association studies on homogenous groups of patients, especially where the impact of polymorphisms on psychopathology of schizophrenia is assessed. Genes and their polymorphisms were selected for the study based on an extensive literature review. As described in the introduction, many authors have emphasized the potential role of IL-2, IL-6, and TNFα in the development of schizophrenia.
The functional significance of selected
IL-2,
IL-6, and
TNFα gene polymorphisms has been previously determined. An
IL-2 genetic T→G polymorphism is located 330 bp upstream to the transcription start-site (within the region containing the NFAT transcription factor binding site).
27 Hoffmann et al.
28 reported that the GG genotype is associated with a high level of IL-2, whereas TT and GT genotypes are linked to reduction in the production of this cytokine. Four polymorphisms in the promoter region of the
IL-6 gene have been described: at positions −597 G/A, −572 G/C, −373 A/G, and −174 G/C. The promoter region from −180 to −123 is crucial for transcriptional induction of
IL-6 gene in response to several factors such as viruses or cytokines such as IL-1, TNFα.
29 Polymorphism of −174 G/C has an influence on the IL-6 expression level, which is increased by the presence of allele G, as compared with the allele C. However, some authors reported no linkage between the concentration of IL-6 and the polymorphism at position −174.
30 A
TNFα promoter polymorphism at position -308 (−308G/A) influenced gene expression, and allele A is, according to some authors, associated with a higher level of TNFα production.
31 However, other published reports indicate that allele A reduces TNFα expression or there is no difference in the TNFα mRNA levels between −308A and −308G alleles.
32,33Epidemiological studies indicate that maternal infections (influenza, rubella, toxoplasmosis) during the first and the early second trimesters of pregnancy are linked to greater risk for schizophrenia development.
34–36 The precise neurobiological mechanism explaining those increased risks in relation to infections is not currently known. The role for cytokines and an impaired immune response to these infections during the critical period of brain development is commonly considered.
37 Results from studies conducted among pregnant women and in animal models indicate that the concentrations of cytokines such IL-1β, IL-6, and TNFα, which regulate normal brain development, are increased after infection.
38 Moreover, dysregulation of maternal cytokines may be responsible for behavioral deficits and/or cognitive functioning in offspring.
3 Taking into account that mRNAs expression for cytokines in the CNS is developmentally regulated, altered level of cytokines, including IL-6 and TNFα after maternal infections may lead to abnormal cortical development, and, ultimately, schizophrenia.
38 We hypothesized that IL-6 and TNFα gene polymorphisms that contribute to changes in cytokine levels may impair the immune response to infections and also affect the development of the normal brain.
In this study, we have found statistically significant differences in the frequency distribution of genotypes and alleles for the
IL-2 polymorphism between paranoid schizophrenia patients and controls. The presence of genotype TT and allele T correlates with an increasing risk of paranoid schizophrenia. Our results are in line with the results of Schwartz et al.,
39 who found a significant association of the
IL-2 −330 TT genotype with schizophrenia in a German population.
It is known that IL-1, IL-6, and TNFα protect neurons against the toxic effects of such factors as β-amyloid peptide or N-methyl-d-aspartic acid (NMDA).
40 On the other hand, studies conducted in animal models have shown that chronic stimulation of TNFα exert a negative influence on the neurons’ viability.
41 McGuire et al.
42 have found that TNFα is toxic to embryonic mesencephalic dopaminergic neurons. Currently, we have found statistically significant differences in genotype and allele layout for the
TNFα polymorphism between patient and control groups. Genotype AA and allele A (potentially connected with higher production of this cytokine) were over-represented among schizophrenic patients when compared with controls. Our results correspond with those observed by other authors, with an increased level of TNFα in serum and also an increased TNFα mRNA level in the prefrontal cortex of patients with schizophrenia.
43,44 Polymorphism −308G/A in the
TNFα gene has also been examined in other populations. An association between this polymorphism and schizophrenia was recorded, for example, in the Italian population.
45 However, most studies reported no association between
TNFα −308G/A polymorphism and schizophrenia.
46–48Similar to Liu et al.,
6 we did not find any significant differences in the occurrence of
IL-6 −174 genotypes between patient and control groups. In our previous study, we found frequent occurrence of the C allele in both schizophrenic men and women, as compared with healthy individuals, but these differences were not statistically significant.
25In the next stage, we conducted haplotype analysis to determine whether combinations of specific alleles is associated with greater risk of developing schizophrenia. It was found that haplotypes CTA and GTA correlate with increasing risk (4.4 times and 5.9 times, respectively) of paranoid schizophrenia development in this Polish population. Kampman et al.
47 investigated the interaction between the polymorphisms in
TNFα (−308G/A) and
EGF (61A/G) genes. They found that schizophrenic patients with a combination of
EGF A/A and
TNF-α G/A or A/A genotypes are characterized by earlier disease appearance than patients not having such configuration. Our results showed that in the group of patients with the earliest age at onset of schizophrenia, haplotype CTA was the most frequent.
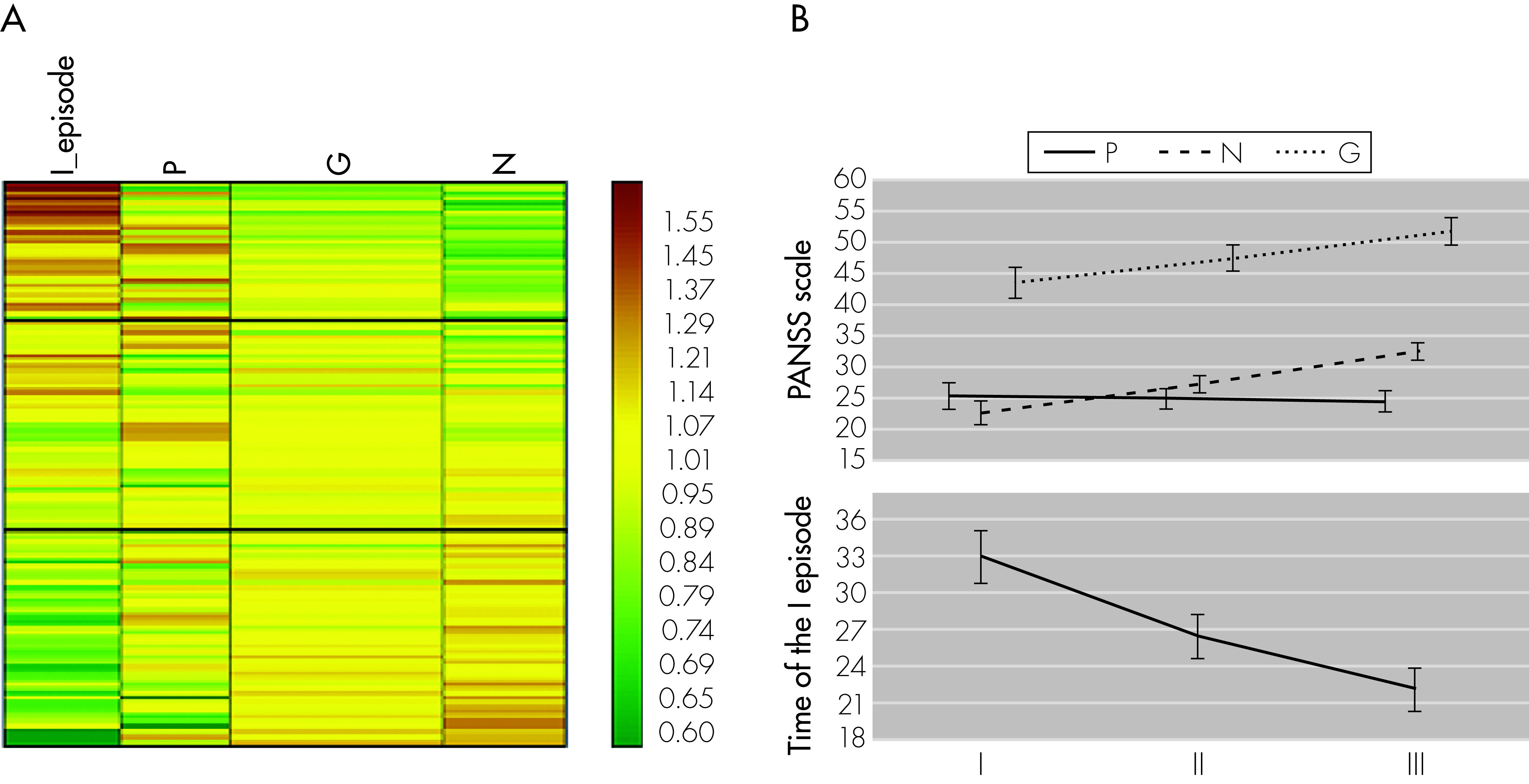
As far as we know, this is also the first study that examines the impact of haplotypes of IL-2, IL-6, and TNFα gene polymorphisms on psychopathological symptoms in patients with paranoid schizophrenia. Because particular schizophrenia subtypes are characterized by different clinical pictures, it is reasonable to perform genetic association studies on homogenous groups of patients, for example, only those with a paranoid schizophrenia diagnosis, as in our case. An association was observed between improvement in the PANSS Negative and General scores, but not in PANSS Positive scores, and CTA haplotype. These results suggest that the CTA haplotype is more likely to contribute to the development of Negative and General symptoms of paranoid schizophrenia. Although IL-6 (−174G/C) polymorphism does not increased susceptibility to schizophrenia, it appears to be important in the context of psychopathology, as in patients with higher intensity of Negative and General PANSS symptoms, the co-presence of allele C (not G) with alleles T and A (for IL-2 -330G/T and TNFα −308G/A polymorphisms) was observed.
Dopamine and serotonin play a major role in mediating the psychotic symptoms of schizophrenia. The dopamine hypothesis of schizophrenia postulates that positive symptoms of schizophrenia may result from excess dopaminergic neurotransmission, particularly in mesolimbic and striatal brain regions, whereas dopaminergic deficits in prefrontal brain regions are responsible for the negative symptoms.
49 According to some authors, IL-2 and IL-6 may be associated with positive and negative symptoms arising in patients with schizophrenia. IL-2 increases dopamine turnover in the prefrontal cortex, whereas IL-6 induces higher activity of serotonin and mesocortical dopamine in the hippocampus and prefrontal cortex.
50 Licinio et al.
51 postulated that IL-2 may cause increase in dopamine neurotransmission in some schizophrenic patients. There is a positive correlation between IL-2 and homovanillic acid (HVA) levels and between HVA and positive symptoms in schizophrenic patients. In contrast, some authors have also suggested a significant inverse relationship between IL-2 level and the PANSS Positive subscale.
50 We have found genotype TT and allele T (connected with lower production of IL-2) with a higher frequency in schizophrenic patients, and this may partly explain decreased dopamine turnover and the severity of negative symptoms in patients with the CTA haplotype. Taking into account that IL-6 also enhance dopamine turnover in the frontal cortex, the additional presence of the allele C for
IL-6 polymorphism (potentially associated with decreased IL-6 expression) may also contribute to the higher intensity of negative symptoms. However, due to the fact that IL-6 has both pro- and anti-inflammatory activities, and molecular mechanism of transcriptional regulation of IL-6 encoding gene is intricate, the results we obtained are difficult to interpret. Such interpretation is also hampered by the fact that cytokines frequently exhibit redundant and pleiotropic effects. Moreover, specific combinations of cytokines may act synergistically or antagonistically, depending on the state of the target cells, and the combination of doses and timing sequence of cytokine release.
4It is postulated that schizophrenia with predominance of negative symptoms is associated with a hypo-dopaminergic state in the prefrontal cortex. According to some authors, TNFα may stimulate the catecholaminergic system, but chronic TNFα release has the opposite effect.
52 We have found genotype AA and allele A (connected with higher production of TNFα) for
TNFα −308G/A polymorphism, similarly to the CTA haplotype, to have a higher frequency in schizophrenic patients, and CTA haplotype was dominant in patients with the highest Negative and General symptoms scores. Naudin et al.
53 previously detected higher level of TNFα in schizophrenic patients, but this did not correspond to the clinical status.
To sum up, the T allele and homogenous TT genotype of −330G/T polymorphism in the IL-2 gene and A allele and AA genotype of −308G/A polymorphism in the TNFα gene may correlate with increasing risk of paranoid schizophrenia development in Polish population, but research on a larger patient group is needed.
Haplotypes CTA and GTA, respectively, are associated with a 4.4- and 5.9-fold increased risk of paranoid schizophrenia development.
Our results support the hypothesis that variations in promoters of IL-2, IL-6 and TNFα genes may contribute to the development and clinical course of paranoid schizophrenia.
The main limitation of the current study is that the relatively small sample size limits the generalizability of our findings. Future replication studies are required to confirm our observations.