To the Editor: A 5-year-old boy of Chinese descent presented to the Early Emotional Development Program at Washington University with a history of bilateral amygdala loss of unknown etiology. His adoptive parents reported that he had been adopted at the age of 18 months from an institutional setting; several months after his adoption, he began to eat less, and, by the age of 22 months, he refused all food. During the first year in his adoptive home, he became aggressive, displaying spontaneous aggression toward his adoptive family. His adoptive parents also described him as being “fearless,” unafraid of strangers or situations that could, and did, cause him harm. We report our clinical observations and the results of testing of this unique child and discuss implications for early brain neuroplasticity.
Intact amygdala functioning has been implicated in a variety of human behaviors, including normal fear response,
1 classical conditioning,
2 pain perception,
3 and emotion regulation. Also, the amygdala is associated with brain systems that serve a number of more complex human abilities, such as social decision-making;
4 trust;
5 and the ability to ascribe various mental states to others, to better predict their behavior
6—the latter, an ability often referred to as possessing an intact “theory of mind,” an ability shown to be absent or impaired in those with autism spectrum disorders. Intact amygdala functioning is thought to be vital component of emotional systems, with the implication being that more complex behavioral functions are laid down developmentally, elaborating on basic emotional processes in which the amygdala plays a significant role. Impaired or poor amygdala function has been implicated in an equally wide variety of psychiatric illnesses and risk states, including psychopathy,
7 anxiety disorders, and mood disorders, early institutional care, and exposure to maternal depression.
8Researchers have made the link between amygdala and behavior using several methods, including neuropathological studies, structural and functional imaging of normal and psychiatric populations, behavioral studies of juvenile and adult primates with lesions of the amygdala and hippocampus, and various assessments of human subjects with known amygdala loss. The latter subjects are most often adult and are often afflicted with lipoid proteinosis or Urbach-Wiethe disease,
9 a rare disorder that leads to bilateral, symmetrical calcifications of the medial temporal lobes in about 50% to 75% of affected individuals. To our knowledge, no one has described the behavior of preschool children with bilateral amygdalar loss.
Despite the lack of descriptions of such children in the literature, some authors have postulated the effects of impaired amygdala functioning on development, using as their source the behavior of juvenile primates who have undergone surgical ablation of the amygdala. They propose that loss of the amygdala early in development could lead to the permanent alteration of normal fear responses.
10 However, research utilizing other animal models suggests that early amygdala loss could potentially be compensated for by other brain regions, given greater neuroplasticity earlier in development.
11 When proposed relationships between impaired amygdala functioning and autism spectrum behavior have been examined in human subjects, subjects with early-onset amygdala loss do show autistic traits
6 at a higher rate than subjects with later-onset amygdala loss,
6,12 providing support for the important early role of a functioning amygdala in organizing later complex social behavior.
We describe the following case of a young boy who presented to the Early Emotional Development Program Infant/Preschool Mental Health clinic at Washington University with a history of unusual behavior and serial structural neuroimaging evidence of progressive, bilateral hippocampal and amygdalar loss. His adoptive parents reported a number of behavioral and developmental concerns, including high levels of unprovoked spontaneous aggression, cognitive delays, and a complete refusal to eat, beginning near the end of his second year.
Case Report
The patient, a 5-year-old, ethnically Chinese boy, presented with a history of aggressive behavior. He was referred by his neurologist where the diagnosis of ESES (electrical status epilepticus of sleep)
13 was made, and a neurodegenerative process was identified with unknown etiology.
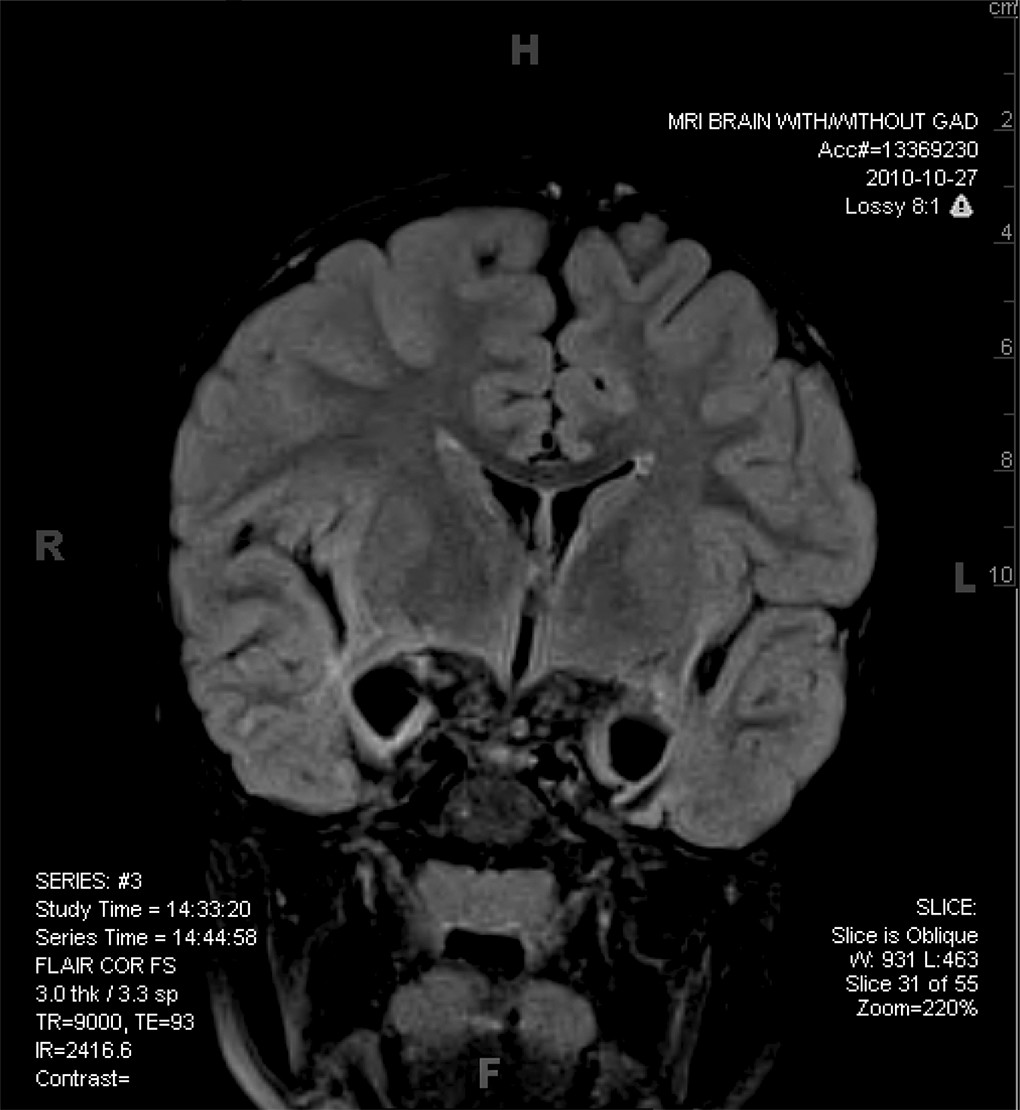
Our patient was found on the streets of Beijing at the estimated age of 2 weeks, after apparently being abandoned by his biological parents. He was cared for in an orphanage for 18 months and was subsequently adopted by an American couple. He presented with some dysmorphic features (a malformed ear and a slightly asymmetrical face), and a weak right arm due to mild cerebral palsy. After being with his adoptive parents for about 5 months, our patient began to refuse to be fed and seemed to have lost all interest in food. Because of concerns about failure to thrive, this ultimately led to the placement of a gastric feeding tube. Aggressive behaviors were another clinical concern and were initially assumed to be related to disordered attachment related to early experience in an orphanage setting. Although there was no clear loss of acquired language or other skills, parental concerns about delayed development resulted in a neurological evaluation, including an MRI, which revealed some atrophy of medial temporal structures initially. A follow-up MRI 2 years later showed progressive, extensive loss of these same structures (
Figure 1).
At the time of the initial interview, the parents reported that our patient had a history of spontaneous, apparently unprovoked aggression associated with inappropriate affect (for example, smiling while pulling out clumps of his sibling’s hair). They also reported no interest in food, no significant mood disturbances, and little-or-no fear. The parents reported both a lack of fear toward strangers and a lack of fear in association with environmental dangers such as falling from heights. According to the parents, he could communicate his needs, but could not speak using words. They reported minimal interaction with other children, very limited shared play, and very simple, mostly a mechanistic, perseverative play, characterized by manipulation of his toys for minutes or even hours. Although his social interactions were not as complex as those of his peers, he would make eye contact readily and seemed to enjoy the company of his family, as evidenced by proximity-seeking and affective reciprocity.
Screening evaluations of autistic behaviors were performed with the M-CHAT and Social Reciprocity Scale (SRS). On the M-CHAT, the patient failed 4 items, and on the SRS completed by the parents, the patient scored well within the clinical range (101 and 104, respectively).
Observation of the patient with his primary caregivers revealed a wide variety of social behaviors. When observed with his mother alone, the patient displayed good eye contact and sharing of play activities. However, when an unknown female examiner entered the room and his mother left the room, the patient immediately dove underneath a table. After few minutes, the boy did begin to interact with the female examiner, rolling a ball back and forth with her and laughing. When the mother returned, the boy was clearly glad to see her, again making good eye contact and smiling.
Discussion
This 5-year-old boy presented with a combination of complex behavioral problems, including developmental delays, markedly impaired expressive language and limited receptive language abilities, refusal to eat, seizures, limited social reciprocity with a history of ritualized social interaction, and aggression. His aggression improved with treatment of his seizures, but was still present—although at a markedly reduced level—at the time of evaluation. His aggressive behavior and his refusal to eat were interesting, given his bilateral amygdala damage, as this kind of lesion has been typically reported to be associated with apathy and hyperphagia in the Kluver-Bucy syndrome.
14Despite the absence of amygdala bilaterally, our patient did evidence anxiety in social settings and did engage in some complex reciprocal social interactions, although it is clear that these interactions were not as well developed and complex as those known in age-matched peers. Despite his adoptive parents’ recounting of overall fearlessness and a lack of stranger anxiety, he did show anxiety when in the presence of strangers, and exhibited this anxiety, for example, by withdrawing completely from the situation, as he did when he hid underneath the table when confronted by a strange examiner.
The presence of social anxiety as well as social competence in our patient argues against the notion that an intact amygdala is required for these complex behaviors. Our patient did clearly exhibit some impaired social interactions, but he was able to interact meaningfully with his caretakers, show preference for primary caregivers, and even show shared, friendly play with a stranger. The conservation of complex emotional behaviors despite the nearly complete loss of the amygdala bilaterally argues for the plasticity and redundancy of the cortical substrates that serve emotional processing in the human brain. This case shows the importance of early childhood competency in emotional processing by showing how robust the systems serving this ability must be, allowing remarkable adaptation even in the face of radical structural abnormalities in the brain. Findings suggest that compensatory mechanisms are possible in the brain emotion system early in life.