Apathy refers to a loss of motivation, in combination with impairments of behavioral, cognitive, and emotional aspects of goal-directed behavior.
1 Individuals with apathy can be misperceived as “lazy” because of difficulty marshaling the effort required to initiate and complete activities. Apathy is seen under various medical or psychiatric conditions, such as traumatic brain injury (TBI),
2 schizophrenia (SZ),
3 Alzheimer’s disease (AD),
4 and Parkinson’s disease (PD).
5 TBI apathy among TBI survivors may be as high as 71%,
2 and can emerge immediately after a TBI or up to several years later.
6–8 The negative symptoms of schizophrenia include apathy. Those with severe and enduring negative symptoms might represent a homogeneous subgroup, termed the “deficit syndrome” of schizophrenia (DSZ), characterized by restricted affect, poverty of speech, anhedonia, diminished sense of purpose, and social withdrawal.
3,9 Previously, we found similar levels of apathy among patients with DSZ and TBI survivors selected on the basis of prominent apathy symptoms. However, compared with those with TBI, the DSZ group showed higher rates of other negative symptoms, including anhedonia, blunted affect, and alogia.
10 Those findings suggest that individuals with DSZ have a constellation of apathy symptoms that differs from that of TBI patients, and possibly associated with different neuroanatomic correlates.
Numerous MRI studies have demonstrated broad regional brain volume reductions after TBI.
11–21 These changes are thought to result from the acute injury, as well as delayed neuronal death attributable to more widespread processes that occur after TBI.
18,21 Likewise, structural MRI studies have shown volumetric differences in the prefrontal and temporal regions among DSZ patients relative to healthy controls (HCs),
22–24 and we previously found gray matter (GM) volume reductions among adults with DSZ in the insula and superior temporal, superior frontal, and fusiform gyri.
25Cognitive performance is also substantially affected in both TBI
20 and DSZ.
26 Specifically, DSZ patients show poorer performance on a variety of cognitive tests, including memory, as compared with HCs, and the severity of negative symptoms (psychomotor poverty, alogia) significantly relates to the amount of decrement in performance on cognitive tests.
27,28 A relationship between cognitive deficits, including verbal memory, and apathy symptoms has also been shown among TBI survivors.
29 We have been interested in delineating the clinical and biological correlates of apathy across specific neuropsychiatric diseases in order to assess the validity of its syndromal construct.
10 This interest has been initially driven by our study of DSZ and the need to better define the biological characteristic of this syndrome, including brain structure abnormality. We thought that TBI apathy would constitute a “model” for DSZ because of the high prevalence of apathy among TBI survivors and because TBI apathy could be more easily related to localized brain abnormality with potential pathophysiological relevance to DSZ.
Therefore, the goals of the present exploratory study were to 1) compare brain morphology among DSZ, TBI, and HC participants; and 2) assess whether specific differences in cortical thickness or regional brain volume correlate with the severity of apathy and performance on tests of cognitive functioning in TBI and DSZ.
Methods
Study Participants
A group of 10 TBI patients were recruited from the Brain Injury Clinic at Johns Hopkins Hospital and from local rehabilitation centers. Injury details were obtained from the patient, a collateral informant when available, and medical records. All TBI patients survived a closed head injury secondary to either a motor vehicle accident (N=8) or a fall (N=2) sustained at least 18 months (mean: 72 [standard deviation {SD}: 47] months; range: 18–157 months) before study participation. Based on available data, including Glasgow Coma Scale scores and the duration of loss of consciousness, 6 individuals were rated as having sustained a severe TBI; 2 had a moderate brain injury; and 2 had a mild TBI. These participants all met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for a “personality change secondary to TBI, apathetic subtype.”
A group of 15 adults with schizophrenia, diagnosed according to DSM-IV criteria, were recruited from outpatient clinics, inpatient services, and psychiatric day hospitals affiliated with the Johns Hopkins University and Hospital. All participants in the TBI and DSZ groups met criteria for the “deficit syndrome” on the basis of the Schedule for Deficit Syndrome (SDS).
30 None of the DSZ or TBI patients met criteria for either a mood disorder or substance abuse/dependence during the preceding 6 months.
The HC group comprised 47 healthy adults recruited for a study of normal aging from the Baltimore metropolitan region via random-digit dialing or telephone calls to randomly selected telephone numbers in the residential telephone directory. Exclusion criteria included any reported history of neurological disorders, neurodegenerative disorders, psychiatric illness, and current alcohol/drug abuse or dependence. These studies were reviewed and approved by the Johns Hopkins Hospital Institutional Review Board, and all participants provided written informed consent before participation.
Procedure
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) was used to diagnose both schizophrenia and personality change secondary to TBI. The presence and severity of apathy in TBI and DSZ participants were assessed based upon the Apathy Evaluation Scale, Clinician Version (AES–C).
31 The SDS and the AES–C were administered by one of two board-certified psychiatrists (NC and VR). Acceptable interrater reliability for the SDS (κ=0.73) was established between raters. The AES–C has been shown to have good internal reliability, test–retest reliability, and interrater reliability (reviewed by Clarke et al.
32). We used the Scale for the Assessment of Positive and Negative Symptoms (SAPS and SANS) and the Hamilton Rating Scale for Depression (Ham–D) to assess severity of psychopathology and depressive symptoms. The National Adult Reading Test–Revised Version (NART–R) was used to estimate premorbid IQ.
33The HC group was administered diagnostic and clinical assessments, including the Schedule for Clinical Assessment in Neuropsychiatry (SCAN) interview,
34 review of medical history, and physical and neurological examinations. Whereas the majority of TBI survivors and HCs underwent physical and neurological examination, psychiatric interview, a brain MRI scan, and cognitive assessment on the same day, most of the DSZ patients needed several days (mean: 4.1 [7.5] days) to complete these procedures.
Cognitive Measures
Each participant was administered a brief neuropsychological test battery detailed in our previous study.
20 This battery assessed functions including psychomotor speed and dexterity, psychomotor processing speed, auditory divided attention, letter–word fluency, visual construction, verbal learning and memory, visual learning and memory, and concept-formation.
MRI Acquisition and Pre-Processing
All participants underwent brain MRI on the same 1.5-tesla GE Signa scanner (Milwaukee, WI). We acquired 124 contiguous 1.5-mm, 3-D SPGR slices in the coronal plane. The parameters were the following: repetition time: 35 seconds, echo time: 5 seconds, flip angle: 45°, and image matrix: 256×256. Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer Image Analysis Suite, Version 5.0 (
http://surfer.nmr.mgh.harvard.edu/fswiki). Detailed methodology of the Freesurfer program is available elsewhere.
35Statistical Analysis
One-way analysis of variance (ANOVA) was used to compare all three groups with regard to age, years of education, estimated full-scale IQ, and cognitive test results. The Scheffé test was used for post-hoc pairwise comparisons. Chi-square tested for differences in sex and handedness between the TBI and DSZ groups. Finally, independent-sample t-tests assessed for differences between the TBI and DSZ groups with respect to AES scores, SANS/SAPS total scores, SANS subscale scores, and Ham–D scores.
Subcortical and cerebellar volumes were compared across the three diagnostic groups via analysis of covariance (ANCOVA), with diagnosis as a between-subject factor, and age, gender, and intracranial volume (ICV) as covariates. Scheffé tests were used for post-hoc group comparisons. When significant volumetric changes were found between either patient group and the HC group, we calculated partial correlation coefficients between the region-of-interest (ROI) volumes of that patient group and AES scores and neurocognitive measures while controlling for age, sex, and ICV.
We used FreeSurfer’s general linear model with a 10-mm, full-width, half-maximum Gaussian kernel to compare cortical thickness across groups (DSZ versus HC, TBI versus HC, and DSZ versus TBI) and to correlate AES ratings and neurocognitive scores, while treating age and sex as nuisance variables. When significant cortical thickness changes were observed between either (or both) clinical groups (i.e., DSZ or TBI) and the HC group, additional correlation analyses were conducted independently for each patient group. Monte Carlo simulation was used to correct multiple comparisons.
Separate associations were explored between apathy symptoms and cognitive performance for each patient group by calculating partial correlation coefficients between AES scores and cognitive measures while covarying for age and sex.
The statistical significance level was set at p <0.01 for most statistical analyses, to control for type I error. Based on the anticipated loss of statistical power secondary to modest sample sizes of the patient groups, the statistical significance level was set at p <0.05 for t-tests and post-hoc comparisons (i.e., Scheffé’s test and Monte Carlo simulation). All statistical analyses were performed with SPSS Version 19, with the exception of the cortical thickness analyses, which were conducted in FreeSurfer.
Discussion
To our knowledge, this is the first study to demonstrate an association between brain structure and post-TBI apathy. We observed volume reductions of the hippocampus, the thalamus, and the brainstem among TBI survivors. Among these patients, greater reductions of left hippocampal volume correlated strongly with more severe apathy, and the severity of apathy was negatively associated with performance on measures of verbal learning and memory in TBI patients. In contrast, subcortical volume change was not seen in DSZ patients as compared with HCs. Taken together, these findings suggest that apathy resulting from TBI might have a different neural basis from apathy resulting from schizophrenia. The hippocampus plays a critical role in memory function, including verbal and narrative memory.
7,36,37 Ariza and colleagues
17 reported an association between verbal memory deficits and left hippocampal atrophy in TBI. Andersson and Bergedalen previously reported a relationship between cognitive deficits, including verbal memory, and apathy symptoms among TBI survivors.
29 Although we acknowledge that apathy may affect patients’ cognitive performance, it could be possible that, taken together with our data, the lesions to the left hippocampus might be causally related to the development of apathy and verbal memory impairment in TBI. However, given that subcortical and cortical areas of the brain are interconnected both structurally and functionally, it is possible that, in TBI, the cortical areas associated with the development of apathy could be functionally impaired as a result of structural lesions of subcortical regions such as the thalamus, the brainstem, or the hippocampus. An apathy syndrome has been previously described both after thalamic stroke
38 and brainstem lesions,
39 indicating that such a mechanism is possible. In addition, or as an alternative to, the above explanations, our data showing a correlation of left amygdala volume with AES score (r = −0.81 [p=0.03], controlling for age, gender, and ICV) suggest, if confirmed in a larger sample, a role of the left amygdala−hippocampus complex in mediating apathy symptoms in TBI.
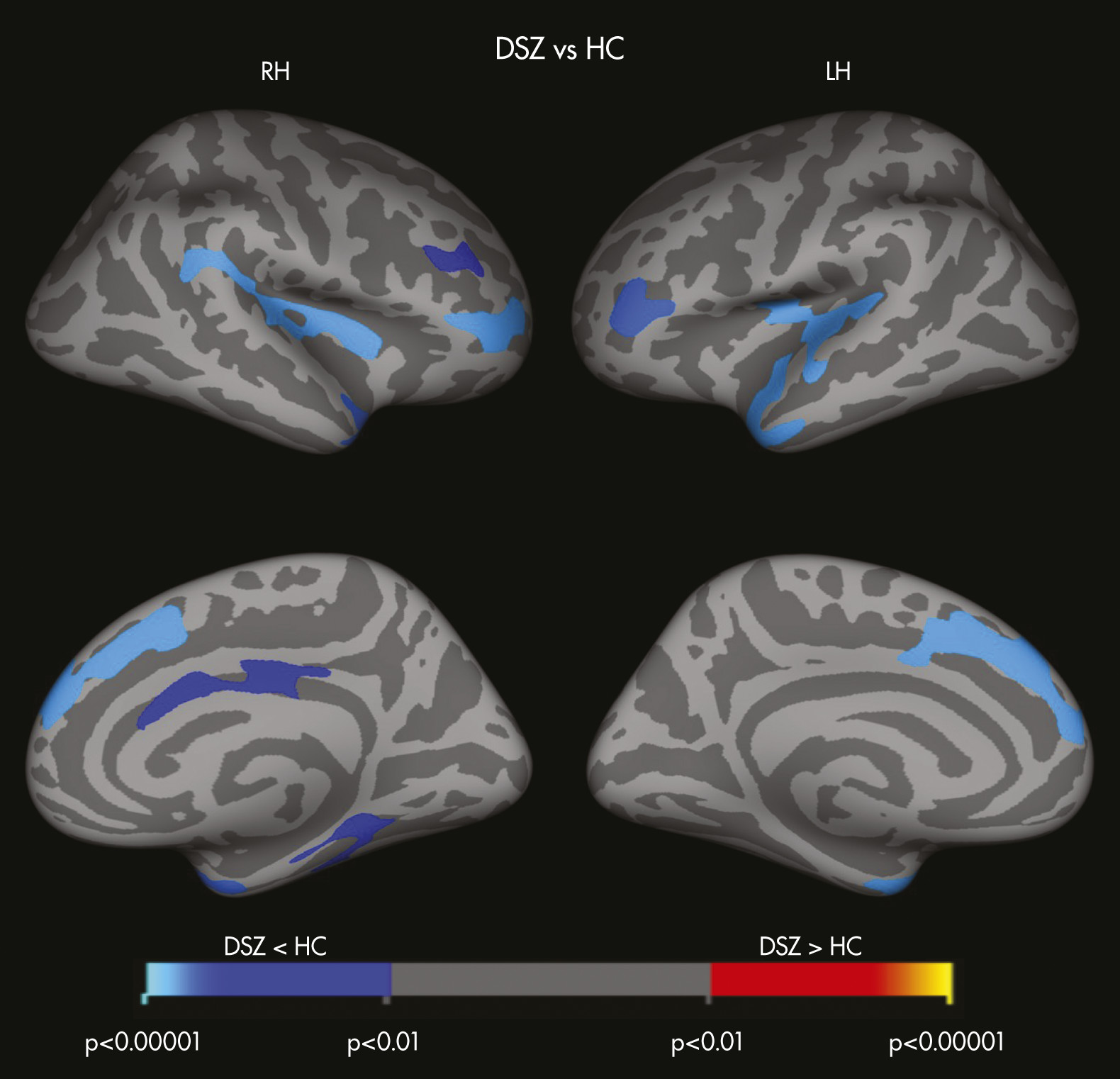
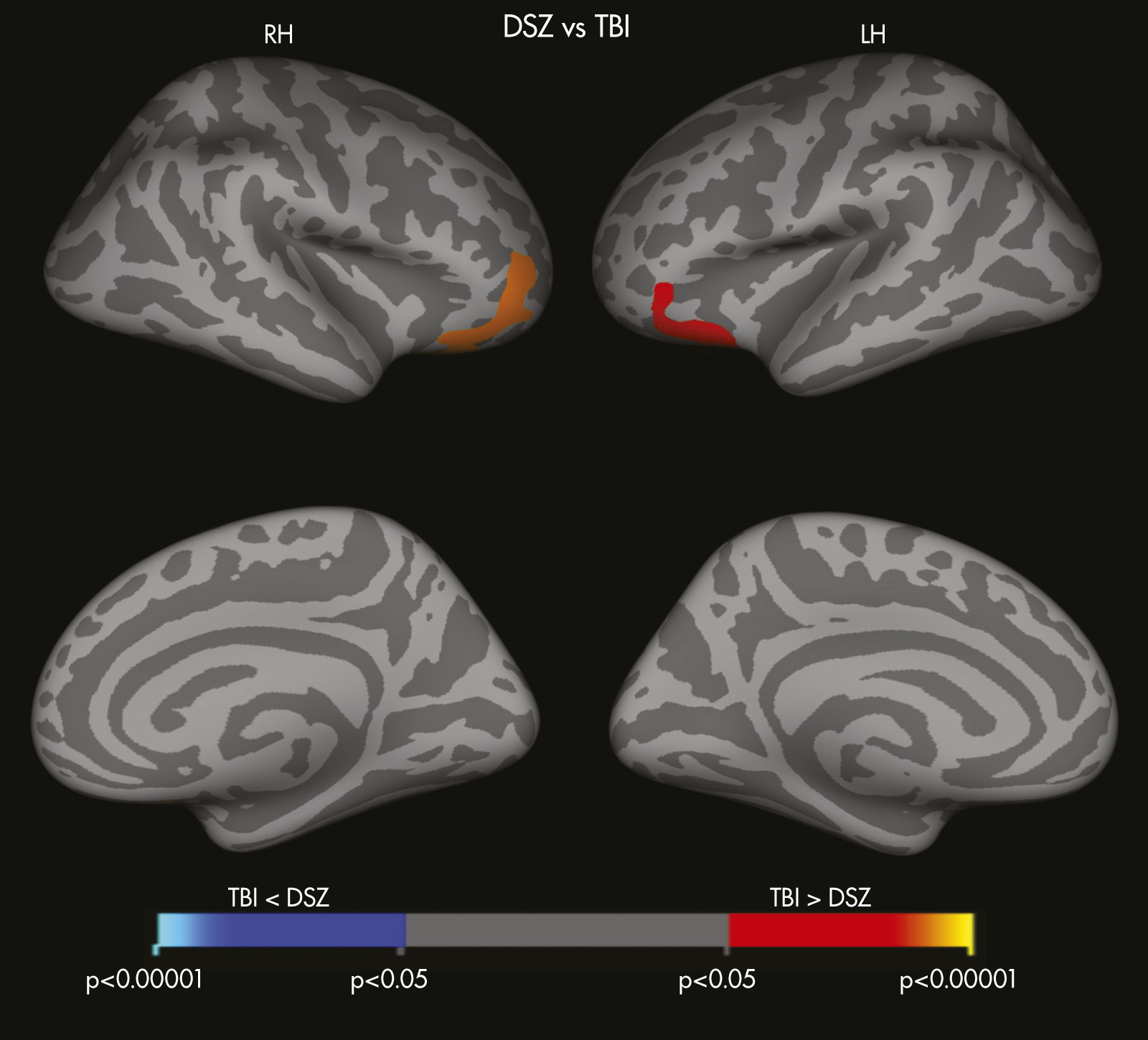
Findings in this study also highlight differences in structural and functional brain changes between TBI survivors and patients with DSZ. Whereas TBI survivors exhibited several regions of subcortical volume loss as compared with HCs, patients with DSZ demonstrated thinner cortices, particularly in fronto-temporo-paralimbic regions, as compared with HCs. Patients with DSZ also showed significantly reduced cortical thickness of the pars orbitalis bilaterally and lateral orbitofrontal cortices (LOFC) relative to the TBI group. Lesions to the LOFC, one of the frontal lobe areas that is connected with subcortical limbic regions, results in personality blunting and change, possibly due to the absence or impairment of limbic affective input.
40 Furthermore, LOFC is particularly sensitive to the implementation of reversal learning,
42 and schizophrenia patients with “residual” negative symptoms are more likely to fail the set-shifting stage of the reversal learning task.
43 Our results revealed that, DSZ patients completed significantly fewer categories on the M-WCST, compared with TBI survivors with equally severe apathy.
Although Roth and colleagues
44 reported smaller bilateral frontal lobe volumes among SZ patients with severe apathy, based on the SANS Apathy subscale, as compared with HCs, we failed to find any significant correlations between structural brain changes and apathy in DSZ patients. A larger sample size may be required in order to replicate the findings of Roth and colleagues with respect to the relationship between apathy and frontal cortex integrity.
It has been previously proposed that apathy may not be a single syndrome, but one that could be divided into subtypes on the basis of the underlying neural mechanism affected by the pathologic processes.
40 An example of such an approach, and relevant to our findings involving lateral orbitofrontal cortices in DSZ, can be seen in the apathy associated with frontal system dysfunction. Damage to each of the motor and behavioral cortical–subcortical circuits
41 that involve frontal lobe, striatum, globus pallidus, substantia nigra, and thalamus, possibly give rise to different forms of apathy. Given that we thought that TBI apathy would constitute a “model” for DSZ because TBI apathy could be more easily related to localized brain abnormality with potential pathophysiological relevance to DSZ, our data suggest that the pathophysiology associated with TBI is different from that involved with DSZ.
Study Limitations
First, this study is limited by the small sample size, as described above, and our findings need to be validated with a larger number of participants. Second, since the TBI patients of this study were heterogeneous in terms of the severity of TBI and the time since head injury, such heterogeneity may have affected their neurocognitive performance or the severity of apathy. Because of such heterogeneity, we were unable to detect differences between patient groups on some cognitive domains. Third, we were unable to address the question as to whether patients in either group showed signs of apathy before they were injured or developed SZ. Fourth, although we excluded patients who had alcohol/drug abuse or dependence within the past 6 month before the study, we were not able to evaluate the lifetime history of alcohol/drug problems, which may have confounded our results. Finally, we did not have reference patient-groups, such as TBI patients without apathy or SZ patients without apathy.
In conclusion, unique changes in brain morphology differentiated our TBI survivors from patients with DSZ, despite similar degrees of apathy across groups. In TBI survivors, greater apathy was associated with reductions in left hippocampal volumes and more severe verbal memory deficits, suggesting that in TBI, as opposed to DSZ, the development of an apathy syndrome may be precipitated by damage to particular subcortical brain regions and may accompany specific cognitive deficits.