Attention-deficit hyperactivity disorder (ADHD) is a disorder primarily characterized by inattention, impulsivity, and hyperactivity, with a prevalence of 5.3%.
1 It has an estimated heritability of approximately 76% and is thought to be a complex, polygenic disorder.
2Although the etiology of ADHD is not fully understood, there is evidence that dysregulation of the central noradrenergic system may be involved in the pathophysiology of ADHD.
3,4 It has been suggested that norepinephrine improves attention by stimulating the α2-adrenergic receptors present in dopamine-containing neurons, which increases the signal-to-noise ratio of these neurons.
5–7 The α2A-adrenergic receptor gene (
ADRA2A), located on chromosome 10q24–26, has been suggested as one of the candidate genes associated with ADHD. The −1291 C-to-G single nucleotide polymorphism (SNP), which creates an
MspI site (rs1800544) in the promoter region of
ADRA2A8 and a C-to-T polymorphism in the 3′ untranslated region (3′-UTR) known as the
DraI site (rs553668),
9 are the two major polymorphisms investigated in relation to the disorder.
In an effort to elucidate the relationship between genetic factors and the associated neurobiological substrates, recent studies have combined genetic and neuroimaging or neuropsychological data.
10,11 Our recent genetic imaging data suggested that subjects with ADHD who carried the C allele in the
MspI polymorphism showed reduced perfusion in the orbitofrontal regions, as compared with those without the C allele.
12 Also, we have suggested a possible role of the
DraI polymorphism of ADRA2A in cognitive impairments in ADHD, such as increased variability in response-time performance.
13 Previous diffusion tensor imaging (DTI) studies of subjects with ADHD have reported the presence of white-matter abnormalities such as increased functional anisotropy (FA) or decreased FA values in several brain areas.
14 We postulate that this lack of consistency might be associated with the heterogeneous composition of ADHD subjects, which is partially attributed to the genetic heterogeneity underlying this disorder.
However, the association between white-matter connectivity and genetic polymorphisms, specifically
ADRA2A, in ADHD, has not been previously investigated. Also, no study had integrated DTI, genetic, and neuropsychological data from subjects with ADHD. Therefore, this study aimed to examine the association between the
MspI and
DraI polymorphisms of
ADRA2A and white-matter connectivity and attentional performance in children with ADHD. Based on previous studies, which reported that norepinephrine improved attention and working memory by recruiting the prefrontal cortex through actions at postsynaptic α
2A-adrenergic receptors,
5–7 we postulated that ADRA2A would have effects on attentional performance and white-matter abnormalities, particularly in frontal regions.
Methods
We recruited 53 drug-naïve children with ADHD from the child psychiatric clinic of the Seoul National University Hospital in South Korea. The recruited children were between 6 and 18 years old and had been given a diagnosis of ADHD according to the DSM-IV criteria as ascertained by a child psychiatrist. Exclusion criteria included 1) presence of any other mental disorders, except for mild oppositional defiant disorder or an anxiety disorder not requiring medication; 2) history of neurological illness; 3) IQ below 70; 4) presence of learning disabilities; 5) any history of substance abuse; and 6) any previous administration of methylphenidate (MPH). To diagnose ADHD and any comorbid disorders, we used the Korean Kiddie-Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (K-SADS-PL).
15Procedures
Before the administration of medication, the parents of all participants completed the Attention-Deficit Hyperactivity Disorder Rating Scale (ADHD-RS), which is a symptom severity scale, composed of 18 items according to DSM-IV criteria.
16 Parents are asked to determine symptomatic frequency that describes the child’s home behavior over the previous 6 months. All participants completed the Continuous Performance Test (CPT) and DTI, and after that, they received 0.35–1.77 mg/kg/day of MPH for 8 weeks. Doses were adjusted depending on each patient's symptom severity and drug tolerance. After 8 weeks of MPH treatment, the ADHD subjects completed the CPT again.
This study was conducted as part of Health & Medical Technology R&D program and was approved by the Institutional Review Board for human subjects of the Seoul National University Hospital. Parents/guardians provided written informed consent, and the children provided verbal assent to participate in this study.
We used a computerized CPT
17 to measure the neuropsychological functions of the ADHD children. The four variables recorded were Omission Errors (a measure of inattention), Commission Errors (a measure of impulsivity), Response Times for correct responses to the target (a measure of information-processing and motor response speed), and the Response-Time Variability (a measure of variability or consistency of attention.
Genotyping
The ADRA2A polymorphisms were genotyped as previously described by Cho et al.
13 with slight modifications. Genomic DNA was extracted from whole blood according to standard protocols. The detection of a single nucleotide polymorphism was based on analysis of primer extension products generated from previously amplified genomic DNA, using a chip-based, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry platform (Sequenom, CA; details of protocols are available on request.) Genotype analyses were performed blinded to all clinical, neuropsychological, and imaging data.
DTI Imaging Protocol
We obtained images using a cerebral gradient coil and single-shot spin-echo echo planar imaging (EPI) sequence with 3.0T magnetic resonance (MR) scanner (B-factor=1,000 sec/mm2). Images were acquired with repetition time/echo time (TR/TE) of 10,000 msec/90 msec, field of view (FOV) of 240×240 mm2, matrix size of 256×256, slice thickness/gap of 3.5 mm/0 mm, and a total slice number of 38.
In order to obtain information about the direction and size of the anisotropy of diffusion, we obtained 25 MR images with a noncollinear diffusion gradient and a single MR image without the gradient (b=0). From these 26 diffusion emphasis images, six diffusion tensor components (Dxx, Dyy, Dzz, Dxy, Dxz, Dyz) were calculated, using a multilinear equation. Based on the obtained diffusion tensor components, fractional anisotropy (FA) was calculated for each voxel.
Imaging Data Analysis and Statistical Analysis
FA maps were generated using DTI Studio software (Dept. of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD;
http://cmrm.med.jhmi.edu/), and the following image preprocessing steps were performed, using SPM8 (Wellcome Dept. of Cognitive Neurology, Institute of Neurology, University College London, U.K.;
www.fil.ion.ucl.ac.uk/spm/software/). A brain template specific to this study was created based on the age data of our participants. Here, we used the Template-O-Matic toolbox,
18 which provides reference brain imaging data based on the NIH study of normal brain development (N=394; age range: 5–18 years). We then normalized all of the b=0 images to the standard Montreal Neurological Institute space, using this customized brain template, and applied these normalization parameters to the FA maps. The normalized FA maps were smoothed with a 6-mm full width at half maximum isotropic Gaussian kernel. Finally, FA maps were calculated and submitted to second-level group analyses. All image analyses were performed while blinded to all clinical, neuropsychological, and imaging data.
We performed intergroup analyses with voxel-wise
t statistics, using SPM8. We made comparisons between ADHD patients with and without rare alleles (GG versus GC+CC for
MspI and CC versus CT+TT for
DraI). Voxel-level inference was performed at a height threshold of p <0.0005 (uncorrected for multiple comparisons), and cluster-level inference at p <0.05 (extent threshold=100 voxels). We were aware that the application of an uncorrected threshold would limit the impact of study results because it increases the risk of false-positive findings. However, it is worth noting that the majority of the whole-brain studies applied uncorrected (p <0.001 or p <0.005) threshold,
14 because discrete microstructural abnormalities may not be detectable if adopting a corrected threshold with an increased risk of false-negative findings. In this study, we selected an uncorrected (p <0.0005) threshold because of the exploratory nature of this study and the need to balance the risk for type I and type II error.
Group differences in the demographic and clinical variables were computed with an independent, two-sample
t-test, or χ
2 test, or Fisher’s exact test. The statistical analyses were performed with SPSS Version 19.0 (SPSS, Inc., Chicago, IL), with statistical significance defined at an alpha level of <0.05. The association between genotypes at the markers and neuropsychological measures was investigated with additive regression analyses that corrected for age, IQ, and baseline ARS score, using SNPstats.
19 Between-group differences according to genotype were assessed based on the percent change in the CPT measurements
rather than on inherent changes, to exclude the influence of baseline CPT scores on the MPH-induced changes to CPT scores. The significance level was set at p value of 0.025 (0.05/2SNPs).
Results
A total of 45 boys and 8 girls (84.9% and 15.1%, respectively) participated in this study. MspI genotype analysis of ADRA2A found a G/G genotype in 25 subjects (47.2%), a C/G genotype in 21 subjects (39.6%), and a C/C genotype in 7 subjects (13.2%). DraI genotype analysis of ADRA2A found a C/C genotype in 15 subjects (28.3%), a T/T genotype in 14 subjects (26.4%), and a C/T genotype in 24 subjects (45.3%). The distributions of genotypes for the MspI and DraI polymorphisms were consistent with the expected Hardy-Weinberg equilibrium values.
Table 1 shows the demographic and clinical characteristics according to
MspI and
DraI genotypes of the ADHD subjects who participated in this study.
There was no significant group difference in the baseline scores on the continuous performance test according to the
MspI or
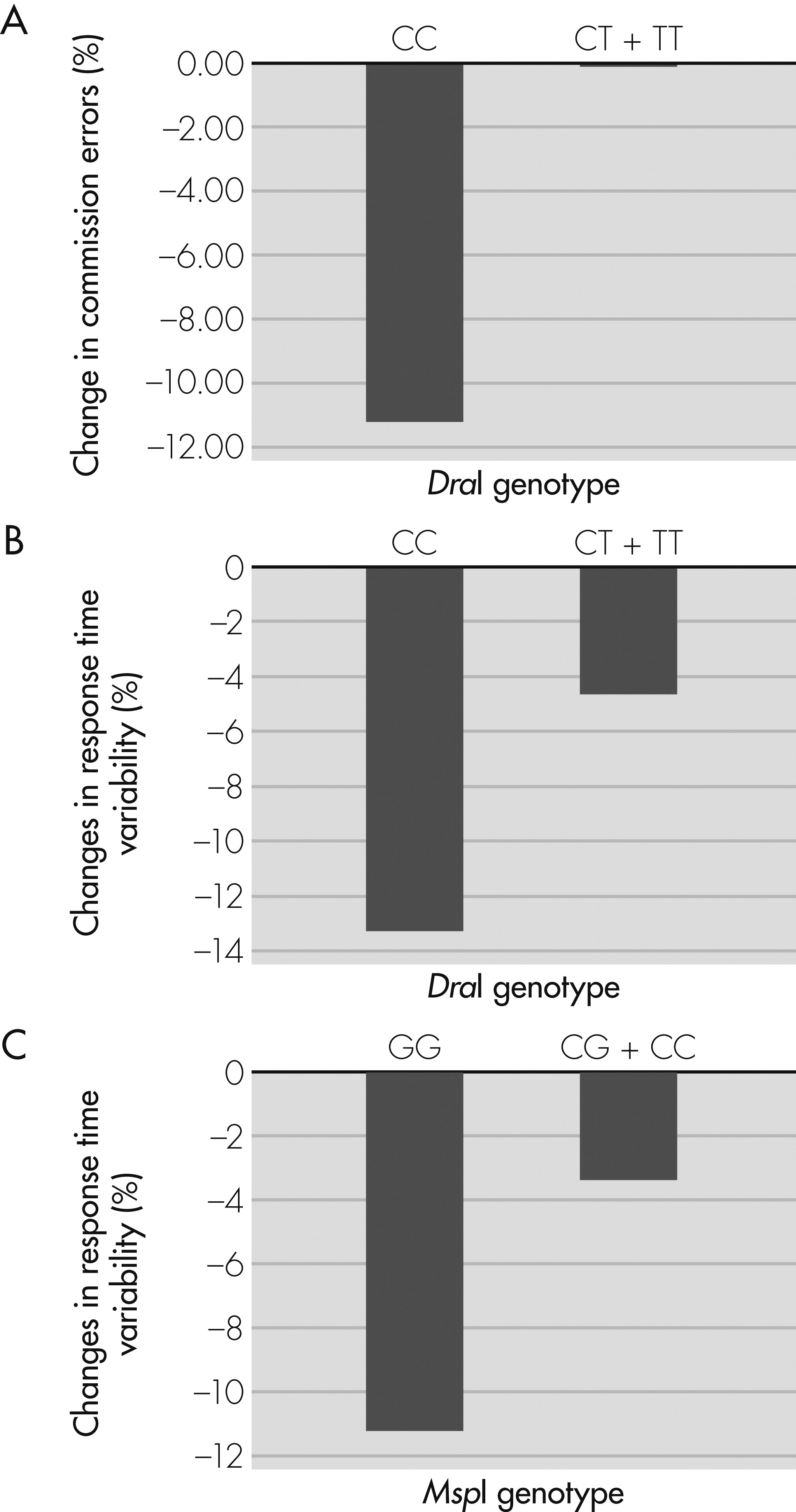
DraI genotypes. However, after 8 weeks of treatment with MPH, ADHD subjects who carried the T allele at the DraI polymorphism showed less improvement in the mean commission error scores (
Figure 1 [A]; p=0.009) and a trend toward fewer changes in the mean response time variability than those without the T allele (
Figure 1 [B]; p=0.069). Also, ADHD subjects who carried the C allele at the
MspI polymorphism showed a trend toward reduced improvement in the mean response time variability than those without the C allele (
Figure 1 [C]; p=0.059;
Table 2,
Figure 1).
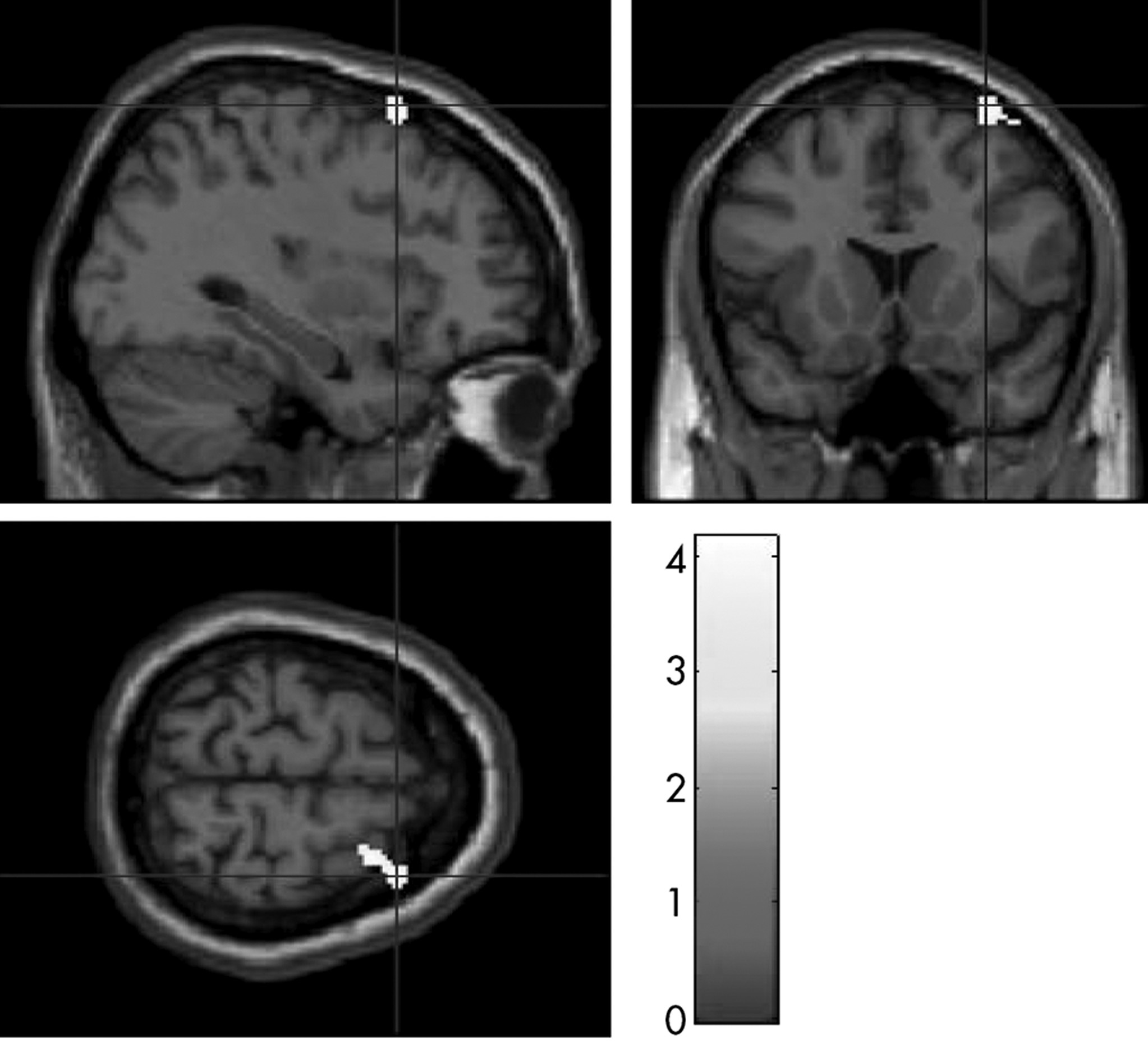
A DTI comparison showed that subjects who carried the T allele at the
DraI polymorphism showed significantly decreased FA values in the white matter underlying the right middle frontal cortex than those without the T allele at both the voxel-level inference (p <0.0005) and cluster-level inference (p=0.047;
Figure 2,
Table 3). Also, ADHD subjects who carried the C allele at the
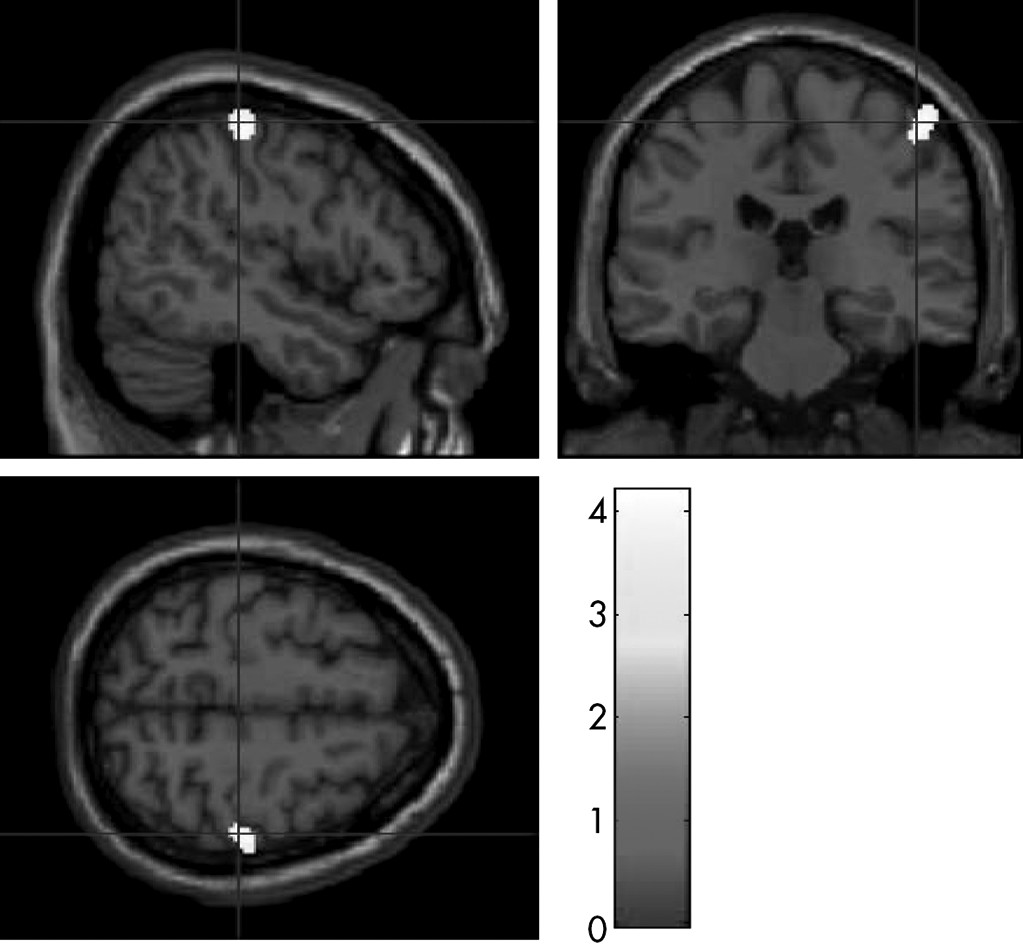
MspI polymorphism showed significantly decreased FA values in the white matter underlying the right post-central cortex than those without the C allele at the voxel-level inference (p <0.0005), but did not show a statistically significant difference in FA values at the cluster-level inference (
Figure 3,
Table 3).
Discussion
To the best of our knowledge, this is the first study to examine the effect of norepinephrine genes on DTI findings as well as the posttreatment neuropsychological characteristics of children with ADHD.
We postulated the frontal region as a highly affected locus in ADHD patients with risk allele of the ADRA2A polymorphism, because noradrenergic transmission in this region has been shown to affect attention by stimulating α
2-adrenergic receptors.
6,21 As expected, we found decreased white-matter connectivity in the right frontal region in subjects with the T allele at the
DraI polymorphism than in those with other genotypes. However, we also found decreased white-matter connectivity in the right parietal region in those with the C allele at the
MspI polymorphism. Pliszka et al.
4 demonstrated that there are two distinct neuroanatomical systems (anterior and posterior) that modulate the attentional networks and play a role in the etiology of ADHD. The anterior system involves frontostriatal circuitry, which is assumed to be associated with executive functions, whereas the posterior system involves the parietal-temporal circuitry, which is thought to be related to the process of selective attention. Our findings of different white-matter connectivity in the frontal and parietal regions according to the
ADRA2A polymorphisms are consistent with the hypothesis of the anterior and posterior attention system.
4ADHD subjects with the T allele at the
DraI polymorphism showed a smaller reduction in the number of commission errors after MPH treatment and decreased white-matter connectivity in the right frontal region than those without the T allele. Commission errors are an indicator of deficits in response inhibition, which is proposed to be the core deficit in ADHD.
20 MPH increases the signal-to-noise ratio of the dopamine-containing neurons in the frontal region by stimulating the α
2-adrenergic receptors, thereby controlling their response inhibition.
21,22 Although the potential functional significance of the
DraI polymorphism is not well understood, the T allele at the
DraI polymorphism may adversely affect the expression and/or function of
ADRA2A, thereby preventing beneficial effect of MPH and resulting in the decreased white-matter connectivity in the frontal region. Although statistically nonsignificant, higher baseline ADHD-RS scores and less improvement in response time variability scores in subjects with the T allele at the
DraI polymorphism also support the idea that this allele is associated with more severe and less treatment-responsive ADHD.
Several limitations may have influenced the findings in this study. First, there was no control group. To determine whether differences in FA values based on ADRA2A polymorphism are specific to ADHD patients, we should have obtained DTI and genetic data from healthy controls. Second, the sample size of the present study was very small for genotypic analysis; thus, the results cannot be generalized to the general population and should be carefully interpreted. Third, there was an unequal distribution of gender in the two genotype groups, which may have affected the neuroimaging results of this study. Finally, the less conservative threshold level used in our SPM analysis (uncorrected p value of 0.0005) had the potential risk of giving unquantified error-control. Therefore, our results should be interpreted with caution, and further work is needed to confirm the findings of this study.
Despite these considerations, this preliminary study provides evidence for the possible role for the ADRA2A in abnormal white-matter integrity and MPH-induced improvement in attentional performance, and supports the noradrenergic hypothesis of ADHD pathophysiology. Further studies should continue to elucidate treatment-related structural–functional changes in the brain, as well as neuropsychological changes according to genetic polymorphisms.