The expansion of radiologic imaging sparked a revolution in the understanding of how brain abnormalities correlate with behavioral disturbances. One area of interest is basal ganglia calcification. The most common presenting symptoms of patients with bilateral basal ganglia calcification (BGC) are movement abnormalities. Although it is less common for BGC to present with isolated psychiatric symptoms, BCG can lead to depression, dementia, or psychosis.
1–3 In this article, we report on a case of new-onset psychotic mania in a 37-year-old man without previous psychiatric history and no neurologic abnormalities, who was found to have bilateral BGC. We then discuss the current understanding of BGC presenting with mania and psychosis, but no neurologic abnormalities, and propose questions for future research.
Case Report
The patient is a 37-year-old white man with no previous medical or psychiatric history, with new-onset psychotic mania. After an argument with his girlfriend over an affair he had had, he developed a marked increase in guilt over his infidelity and his thought process, affect, and behavior changed. His mood became elated and expansive, his speech pressured, and he developed grandiose delusions, claiming he was Christ. His need for sleep was markedly reduced, but his energy increased, leading to excessive and often erratic thoughts and activity. He became atypically impulsive and risk-taking (e.g., citing concerns about the state of the economy, he cashed in his 401k savings and used the money to buy gold). He also developed delusions of reference, interpreting newspaper articles as having specific messages for him and feeling as if songs on the radio were chosen just for him. He slept and ate very little during this time. He denied depressive symptoms. He met DSM-IV-TR diagnostic criteria for bipolar disorder, manic with psychotic features.
4There were no fevers, myoclonus, falls, loss of consciousness, tremors, or other abnormal movements. He denied past history of central nervous system infections or head trauma. He was not taking any medications or supplements. He had used marijuana intermittently as a teenager, but denied recent drug abuse. He had finished high school and worked as a janitor at a local school for the 10 to 12 years before presentation. There was no family history of psychiatric disorders.
On admission, the patient was an athletic man with pressured speech. His conversations dwelled on topics regarding gold, the economy, and impending doom. His affect was expansive, and his thought process was tangential. He had delusions of reference. There were no hallucinations. He was alert and oriented. Attention, memory, language, calculation, praxis, visuospatial, and executive function tests were normal. His physical, including neurologic examination, was unremarkable. There were no abnormal movements or features of parkinsonism.
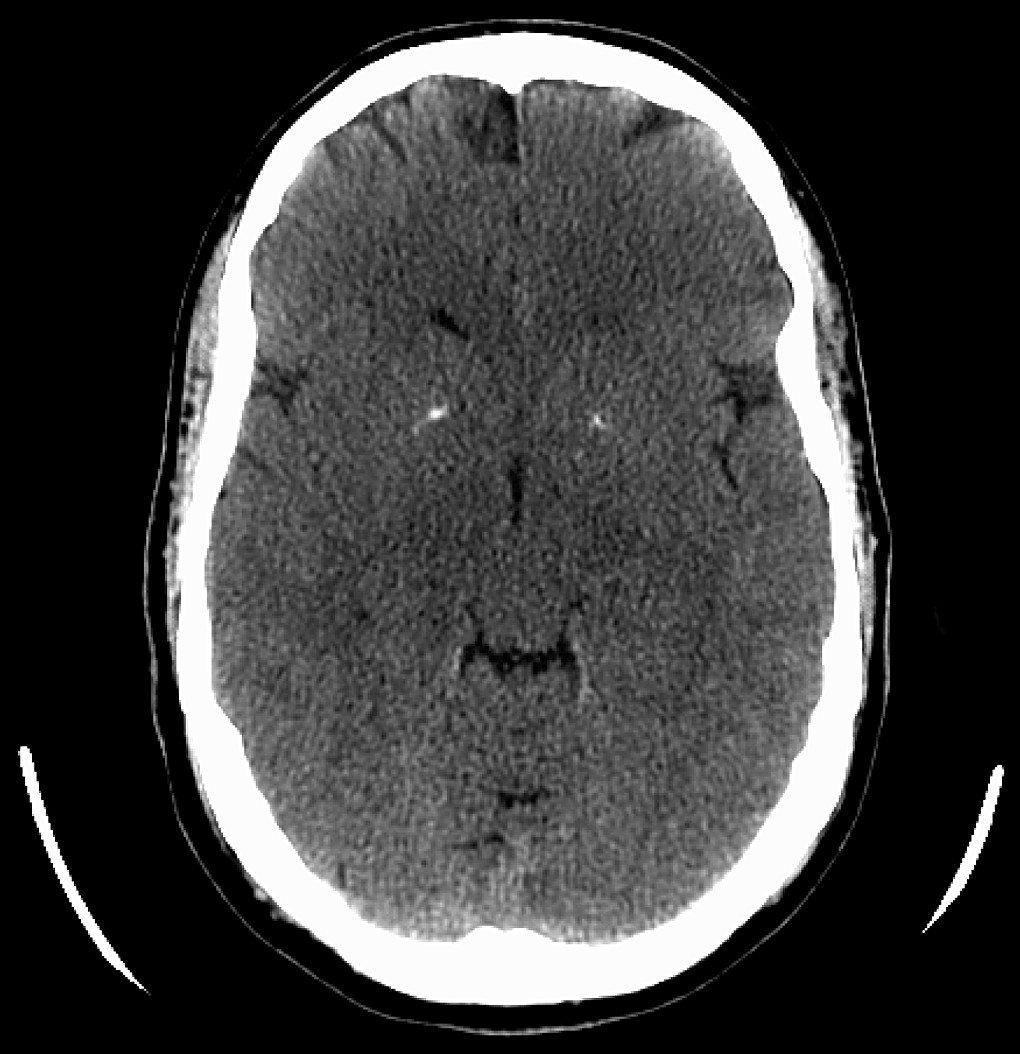
Complete blood count, basic metabolic panel, liver function tests, TSH, CRP, ESR, were unremarkable, with negative RPR. Serum calcium and intact PTH were within normal limits. Urine drug screen was negative. He did have a positive ANA, at 1:30, in a nonspecific speckled pattern. A heavy-metal screen was negative for arsenic, lead, mercury, and cadmium. Computed tomography (CT) scan showed small bilateral basal ganglia calcifications (
Figure 1). He was treated with olanzapine and lorazepam. With treatment, his symptoms improved, and he developed improved insight. The patient was discharged but never followed up.
Discussion
Mania in bipolar disorder typically presents between ages 15 and 30.
3 Because of our patient’s age, work-up for an underlying neurologic disorder ensued. Because most patients with mania do not have BGC, and because many patients with BGC are asymptomatic, we wondered about the correlation between our patient’s syndrome and his evident BGC.
4BGC is a relatively uncommon finding, and bilateral BGC is even more rare. CT imaging is the radiologic modality of choice to identify calcium deposits. A literature review reported the incidence of BGC on a sample of 29,484 CT scans to be 0.93% for all clinical indications.
1 BGC appears to be more common in patients with neuropsychiatric manifestations—it has been found in 9% of CT scans for neurologic or psychiatric indications.
5 Ostling et al.
6 found an incidence of 19% for BGC in mentally healthy 85-year-olds without dementia, but 64% for BGC in 85-year-olds with hallucinations or delusions without dementia. These findings suggest that BGC may increase with aging or psychiatric conditions, but also that BGC may be correlated with increased psychosis. The differential diagnosis of BGC includes hypo- and hyperparathyroidism, AIDS, lead poisoning, hypoxia, and systemic lupus erythematosus, among other conditions.
Overt psychotic symptoms, such as the delusions experienced by our patient, have been reported with BGC disorders such as Huntington’s disease.
1 Given the overlap between the psychotic symptoms present in basal ganglia disorders such as Huntington’s disease and the symptoms seen in schizophrenia, similar mechanisms have been proposed.
Altered dopaminergic transmission in the basal ganglia has been suggested as a mechanism sustaining positive psychotic symptoms in schizophrenia and Fahr’s disease, a disorder of bilateral BGC.
7 A report by Shouyama et al. of a patient with Fahr’s disease with parkinsonian features as well as psychosis revealed hyperperfusion of the lateral temporal lobe via technetium-99 methyl cysteinate dimer single photon-emission computed tomography (SPECT).
8 Several other studies have demonstrated hyperactivation of the temporal lobe in schizophrenia patients with auditory hallucinations as well.
9 The similarities in hyperperfusion of congruent brain regions may suggest potential overlap in brain pathways involved in psychotic patients with BGC and those with schizophrenia.
Cognition, behavior, and movement are dependent on at least five frontosubcortical loop circuits: the motor, oculomotor, dorsolateral prefrontal, orbital frontal, and anterior cingulate circuits.
10 These loops link the basal ganglia and various subdivisions of the prefrontal cortex in segregated but interconnected systems. Whereas two of these circuits compute motor and oculomotor functions, the other three are intimately involved in cognition, behavior, and affect.
10Frontosubcortical circuits provide a framework for the interpretation of psychiatric symptoms in patients with basal ganglia dysfunction.
10 One proposed mechanism includes basal ganglia neuronal degeneration and mineralization that disrupts these pathways, generating psychiatric symptoms. A case report by Saito et al.
11 of a patient with idiopathic BGC and psychosis with drug-induced neurologic abnormalities revealed decreased dopamine uptake in areas of calcification, as well as significant hypometabolism of the right prefrontal, temporal, and parietal cortices. Reversal of this hypometabolism correlated with improvement of the psychotic symptoms after ECT. Decreased dopaminergic transmission in the basal ganglia due to calcification may represent a mechanism for disruption of fronto-subcortical circuits that causes psychosis.
Mood disorders, particularly depressive syndromes, are commonly seen in basal ganglia pathology, as well.
1 It has been estimated that up to 31% of patients with BGC may eventually develop mania.
1 Nevertheless, there are few reports of patients with bipolar disorder and BGC. Several studies have shown increased activation of the basal ganglia in bipolar patients relative to healthy controls.
12,13 Caligiuri et al.
14 showed that manic patients, as compared with depressed patients with bipolar disorder, show altered activity on functional MRI (fMRI) in the globus pallidus (GP), indicating further involvement of this specific nucleus of the basal ganglia in mania.
Interestingly, the GP is the most commonly involved site in BGC.
1 According to classical models, basal ganglia output is mediated by the balance between the direct pathway, which facilitates thalamic and cortical excitability, and the indirect pathway, which reduces this excitability. The GP interna and externa are part of the direct and indirect pathways, and their dysfunction can have a downstream impact in the excitability of the loop circuit. Case reports using positron emission tomography (PET) and SPECT of patients with BGC who presented with cognitive complaints and dementia showed decreased metabolism in the basal ganglia and frontal lobe.
15 Although these cognitive symptoms differ from mania, it remains unknown whether the various clinical presentations of BGC correlate with different brain PET metabolic patterns.
In summary, it is possible that our patient’s mania symptoms resulted from the bilateral calcification of the basal ganglia. It is also plausible that brain calcification may be a marker of focal neuronal injury, rather than being the cause of the neuronal dysfunction. A third hypothesis remains that the BGC had no functional impact and was an incidental finding. The relationship between BGC and manic psychosis requires further investigation and may be a model for investigating the role of subcortical circuits in the pathophysiology of mood and psychotic disorders.