Psychomotor abnormalities are considered key features of schizophrenia and have been reported in every stage of the illness.
1 Birth cohort and prospective family studies have shown that infants who develop schizophrenia as adults reach neuromotor milestones later and have more neuromotor problems in childhood and adolescence than their nonaffected peers.
2–5 Furthermore, a longitudinal study in at-risk adolescents has shown that psychomotor abnormalities increase throughout the prodromal stage.
6 In the diseased state, this disruption of psychomotor functioning is reflected in signs and symptoms such as catatonia, extrapyramidal signs, neurological soft signs, and psychomotor slowing.
7 Although these motor signs and symptoms are found to be highly prevalent in patients diagnosed with schizophrenia, little is known about their course after illness onset.
8The observation that psychomotor dysfunction is already present before and increases around illness onset is consistent with a neurodevelopmental view of schizophrenia, which would predict psychomotor functioning to remain stable in diagnosed patients.
9 The very few studies thus far seem to support this hypothesis with findings of stable or even improving course of motoric neurological soft signs and finger tapping performance in patients with schizophrenia.
10–20 However, some studies report further deterioration of psychomotor functioning after illness onset, which is thought to be associated with a more chronic illness course.
21,22 In addition, associations between symptom severity and psychomotor functioning have been reported in both cross-sectional and longitudinal studies, further challenging the view of psychomotor abnormalities as univocally stable over time.
7,8,10,17,20To minimize the effect of fluctuations in symptoms and medication dose, this study focused on stabilized patients.
The aims of this study were twofold. First, we aimed to evaluate how psychomotor signs and symptoms evolve over a 1-year period in stabilized patients with schizophrenia. Second, we wished to assess the associations between these signs and symptoms.
Methods
Subjects
Patients with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder were recruited in two major in- and outpatient treatment facilities in Flanders (St. Norbertus in Duffel and Onze-Lieve-Vrouw in Bruges). Diagnosis was made based on a clinical interview and all other available information (e.g., medical files and nursing staff observations). The study was conducted in compliance with the regulations of the participating institutions and the Declaration of Helsinki. Inclusion criteria at baseline were subjects between 18 and 45 years of age, a stable antipsychotic medication regimen for at least 2 weeks before testing, adequate knowledge of the Dutch language, and signed informed consent. Patients who were on benzodiazepines, had recently received ECT (within 30 days prior to testing), had antecedents of psychosurgery, or had a comorbid neurological disorder could not enter the study.
At baseline, 105 patients were included in the study.
Materials
General symptoms.
The Positive and Negative Syndrome Scale (PANSS)
23 was used for the assessment of positive and negative symptom severity. The PANSS is a 30-item rating scale that evaluates positive (POS), negative (NEG), and general (GEN) symptoms based on a semistructured interview.
Psychomotor functioning.
To assess a wide variety of psychomotor signs and symptoms, we chose a validated instrument for each of the psychomotor symptoms clusters that have been described in schizophrenia (i.e., catatonia, extrapyramidal signs, motoric neurological soft signs, and psychomotor slowing). To account for the effect of assessment technique, we included rater-based scales and instrumental tasks in the protocol. This resulted in a test battery that entailed six psychomotor measurement instruments [i.e., Bush Francis Catatonia Rating Scale (BFCRS), St. Hans Rating Scale, Salpêtrière Retardation Rating Scale (SRRS), Neurological Evaluation Scale (NES), Copying Lines Task (CL), and Finger Tapping Task (FTT)].
BFCRS.
The BFCRS
24 was used for the assessment of catatonia. It is a well-validated scale that evaluates the presence and severity of 23 catatonic symptoms (excitement, stupor, mutism, staring, posturing/catalepsy, grimacing, echopraxia/echolalia, stereotypy, mannerism, verbigeration, rigidity, negativism, waxy flexibility, withdrawal, impulsivity, automatic obedience, mitgehen, gegenhalten, ambitendency, grasp reflex, perseveration, hostility, and autonomic abnormalities).
St. Hans Rating Scale.
The St. Hans Rating Scale
25 is a scale developed for the assessment of extrapyramidal symptoms. The presence and severity of dystonia, parkinsonism, and dyskinesia were assessed with this instrument. Because of the extremely low prevalence of dystonia and dyskinesia, these symptoms were not considered in further analyses.
SRRS.
The SRRS
26 was used for the clinical assessment of psychomotor slowing. It is a 15-item rating scale, initially developed to evaluate psychomotor slowing in depression. In addition to psychomotor items, this scale contains items focusing on general depressive symptoms (e.g., rumination). Therefore, only the sum of the psychomotor items (i.e., slowing of gate/walking, slowing of movements of the trunk/limbs, reduction in facial movement/expression, speech and voice modulation, and global rating of the inhibition) was used in the analyses instead of the total score.
Neurological Evaluation Scale.
The Neurological Evaluation Scale
27 measures 21 neurological soft signs. For this study, only the motoric subscales were used [i.e., NES_Coordination (NES_COO) and NES_Sequencing NES_SEQ)]. Items are scored with a 0 (no impairment), 1 (slight impairment), or 2 (marked impairment).
CL.
The CL is a computerized copying task designed to delineate slowing in the initiation of movement from slowing in the execution of movement. The stimuli used in this task are simple, straight lines that can be oriented in four directions (vertical, horizontal, and diagonal in two directions). The participant is asked to copy these lines as fast as possible on a sheet of paper divided into 3×4-cm squares and placed on a digitizer. Stimulus presentation starts as soon as the participant touches the “start” circle with the pen tip, located at the bottom left of the square and ends when the participant starts drawing the line. The task consists of 24 trials.
The outcome measures used are initiation time (i.e., the time between the stimulus presentation and the start of the first drawing movement) and execution time (i.e., the time the participant is actually drawing). This task has been used in our research group since the mid-1990s in numerous studies investigating the symptomatology and pathogenesis of schizophrenia, mood disorders, substance use disorders, and eating disorders.
28–30FTT.
The FTT requires participants to press a tapping key attached to a counter as fast as possible with their index finger in a series of 10-second trials. The task consists of five trials, which can be elaborated to a maximum of 10 trials if the tap frequency lacks consistency. The outcome measures are the mean number of taps over the trials for the dominant and nondominant hand.
Procedure
Patients were enrolled in a longitudinal design over a 1-year period with three assessment times: patients who were included at baseline (T1) were contacted for reassessment after 6 (T2) and 12 months (T3). All patients who entered the study at baseline were contacted at T3, regardless of participation at T2. On all time points, participating patients were assessed with the same test battery, which was administered by trained raters.
Assessment time was approximately 120 minutes at each time point.
Data Analysis
All longitudinal analyses were performed by fitting a linear mixed model, with the variable of interest as a dependent variable and time as an independent variable. To account for the relatedness between observations from the same individual, random effects for intercept and slope were added to the model. Significance was tested using a likelihood ratio test, comparing a model with and without the main effect of time.
To calculate the association between the course of the psychomotor measures and between the course of symptom severity and the course of psychomotor functioning, we calculated the change per time unit for each variable under study, separately for each individual. Linear regression models were fit using the value (for the variable of interest) as an outcome variable and time as an independent variable in every individual. In these models, the regression coefficient for time stands for the slope or the change in outcome variable per unit increase in time. These slopes were retrieved for each individual, for each of the variables of interest. Only individuals with a baseline and at least one follow-up measurement were included.
Subsequently, these individual slopes were then used to calculate to what extent the changes over time in the different variables were correlated, using the nonparametric (Kendall-tau) correlation coefficient. Significance level was set at p<0.01.
All statistical analyses were performed in the software package R, version 2.13.1 (
www.r-project.org). Linear mixed models were fitted using the lmer function in the lme4 package in R.
Results
Sample Characteristics
At baseline, 105 patients entered the study. In total, 55 patients (52.4%) completed at least one follow-up session; 45 patients (42.9%) could be retrieved and tested at the 6-month follow-up and 46 patients (43.8%) at the 12-month follow-up; and 36 patients (34.3%) were tested at all three time points. All patients were being treated with antipsychotic drugs at all time points. Mean chlorpromazine equivalent dose did not change significantly over time.
Dropout appeared to be random, as we could not find a significant difference on any of the symptom or psychomotor variables between patients who did and who did not enter follow-up, except for BFCRS score (t=5.534, p=0.009). Patients who dropped from the study had significant higher BFCRS scores at baseline.
For an overview of the demographic and clinical characteristics, see
Table 1.
Longitudinal Course of Psychomotor Functioning
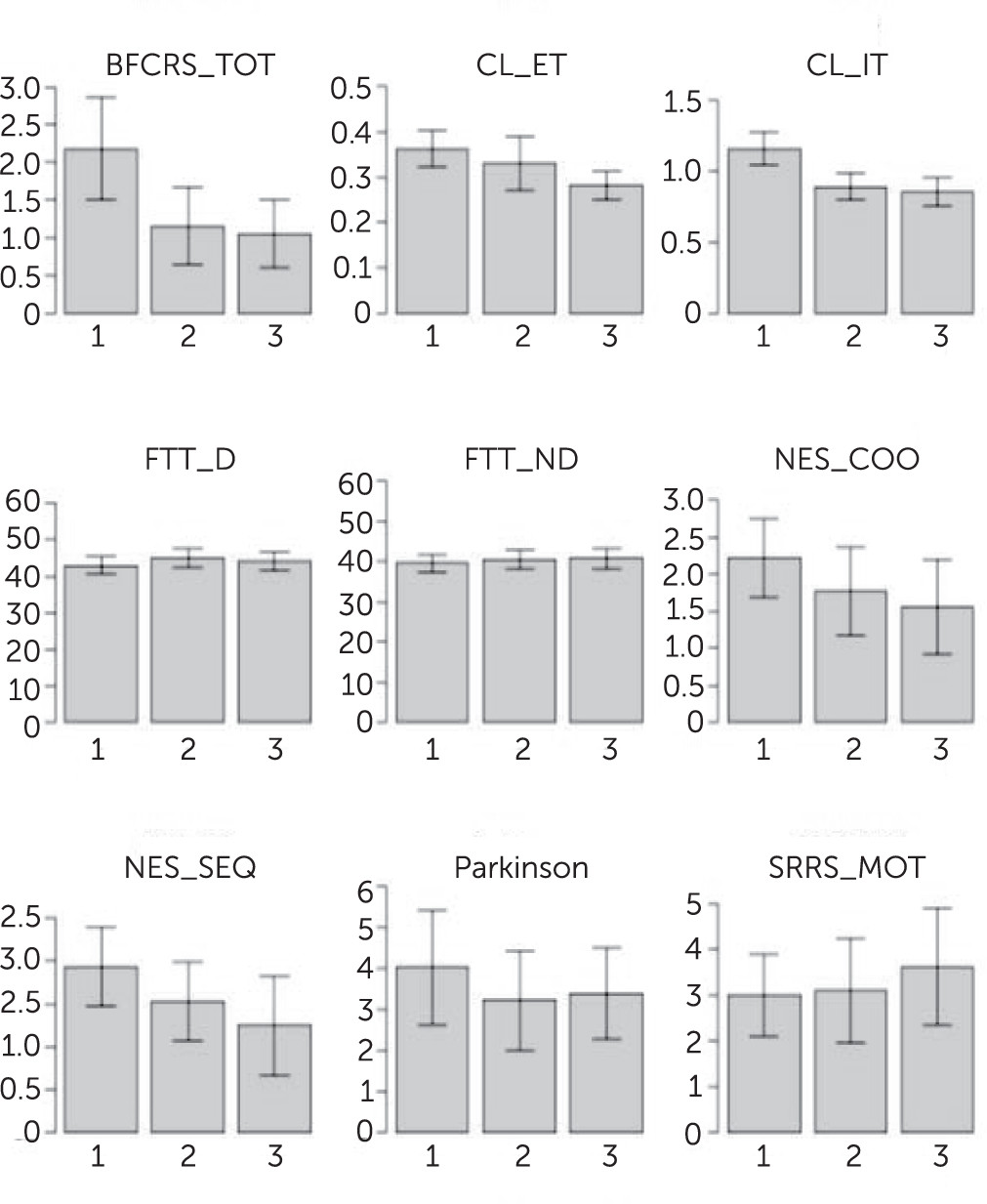
A significant improvement over time was found on the BFCRS (b=−0.592, p=0.003), NES_COO (b=−0.292, p=0.03), NES_SEQ (b=−0.321, p=0.01), and CL performance (IT: b=−0.153, p<0.001; ET: b=−0.037, p<0.001). The change in BFCRS was also reflected in the percentage of patients that had a catatonia rating (i.e., BFCRS>1) at each time point. For the whole sample, these percentages were 72.4% at T1, 54.5% at T2, and 42.5% at T3; for the sample of patients who completed the study, percentages were 72.2%, 54.3%, and 33.3%, respectively, at T1, T2, and T3.
Parkinsonism, SRRS, and FTT performance did not change significantly over the 1-year follow-up period. When controlling for the PANSS subscale scores as covariates in the regression analysis, changes in NES_COO and NES_SEQ were no longer significant. For an overview of these results, see
Figure 1.
Longitudinal Course of Symptom Severity
There was a small, but significant decrease in PANSS_POS (b=−0.798, p=0.04), PANSS_GEN (b=−1.671, p=0.004), and PANSS_total (TOT) (b=−3.167, p=0.008) scores over the follow-up period. Negative symptom severity remained stable over the course of the study.
Associations Between the Psychomotor Measures
To assess the associations between the psychomotor measures at each time point, Kendall-tau correlation coefficients were calculated. Only the data of patients who were tested at all three time points were included in these analyses to consider the same sample in every analysis.
At T1, we found significant associations between parkinsonism and BFCRS, parkinsonism and SRRS, FTT and NES_COO, FTT and NES_SEQ, and NES_COO and NES_SEQ. At T2, significant associations were found between parkinsonism, BFCRS, and SRRS, between CL and NES_COO, and between NES_COO and NES_SEQ. At T3, parkinsonism and BFCRS, parkinsonism and SRRS, BFCRS and NES_SEQ, CL and SRRS, FTT and NES_COO, FTT and NES_SEQ, and NES_COO and NES_SEQ were found to correlate significantly. For an overview of these findings, see
Table 2.
Associations Between the Course of the Psychomotor Measures
To assess whether there is an association between the course of different psychomotor measures, the changes over time of these measures were calculated for every individual using the slopes of a linear regression analysis. Next, Kendall-tau correlation coefficients were calculated between the slopes of the psychomotor variables.
Significant associations were found between the rate of change of BFCRS and FTT, between the rate of change of Parkinsonism and SRRS, and between the rate of change of CL_IT and NES_SEQ (
Table 3).
Associations Between the Course of Symptom Severity and the Course of Psychomotor Functioning
To assess whether there was an association between the course of symptom severity and the course of psychomotor functioning, the changes over time of symptom severity and psychomotor functioning were calculated for every single individual using the slopes of a linear regression analysis. Next, Kendall-tau correlation coefficients were calculated between the slopes for the symptom severity variables and the slopes of the psychomotor variables.
Except for a significant association between the rate of change in SRSS and PANSS_NEG, no associations were found (
Table 4).
Discussion
To the best of our knowledge, this is the first study investigating the longitudinal course of a wide variety of psychomotor signs and symptoms in stabilized patients with schizophrenia. The aim of this study was to assess the course of psychomotor symptoms in schizophrenia over a 1-year period and to assess the syndromic structure of this symptom domain further.
Results show that parkinsonism, clinically observed psychomotor slowing, and finger tapping performance remained stable over a 1-year follow-up period in stabilized patients with schizophrenia, whereas coordination and sequencing deficits, catatonic signs, and psychomotor speed on a copying task improved slightly. However, the improvement over time of coordination and sequencing deficits were no longer significant when controlled for changes in PANSS scores. This finding suggests that the changes in motor coordination and sequencing were the result of changes in overall clinical state rather than of changes in psychomotor functioning as such. This effect of the clinical state on NSS has been described before,
10,20 although it could not be replicated in other studies.
11,12,17 These anomalies might be explained by the existence of subgroups of patients as suggested by Whitty et al.
20 and Chen et al.
21 and should be addressed in further research. Correlations between the course of symptom severity and the course of psychomotor functioning were not found.
Overall, our findings are consistent with the literature considering psychomotor abnormalities to be markers of the neurodevelopmental disease process in schizophrenia.
2–5 The neurodevelopmental hypothesis of schizophrenia claims that the emergence of identifiable schizophrenic symptoms in early adulthood is the end stage of aberrant brain development that starts early in life.
9 This implies that subclinical signs are already present before illness onset, increase around illness onset, and then stabilize. The results of our study provide support for this latter claim. Although previous studies have already shown a stable course of NSS and finger tapping performance after illness onset,
10–20 this is the first study to report longitudinal findings on such a wide variety of psychomotor measures.
Results from this study do not support a neurodegenerative hypothesis. However, this study focused on a rather young population over a relatively short follow-up period. It is possible that neurodegenerative processes only become evident later in life or in a subgroup of patients. In fact, Chen et al.
21 found deterioration of NSS in a sample of patients in late adulthood, and some studies demonstrated a deteriorating course in psychomotor functioning in more severely affected patients.
16 Thus, before the hypothesis of neurodegeneration can be ruled out, further research with longer follow-up periods is needed.
Findings regarding the syndromic structure of the psychomotor symptom domain were mixed. On a cross-sectional level, this study found a rather consistent pattern over time of associations between catatonia, parkinsonism, and psychomotor slowing, as well as associations between sequencing and coordination deficits. These findings are also in accordance with previous cross-sectional work
7,31–33 and with studies examining the association between gross motor activity measures with actigraphy and negative symptoms and schizophrenia subtype.
34,35 As suggested before,
7 the association between catatonia, parkinsonism, and psychomotor slowing may result from the fact that these syndromes were all assessed with the same assessment technique (i.e., rating scales). This could be driven by the fact that there is a partial conceptual overlap (of certain aspects) of these motor syndromes,
36 which may have contributed to certain symptoms being assessed multiple times as part of different motor syndromes. For example, the clinical phenomenon recognized as bradykinesia when investigating extrapyramidal symptoms will also have contributed to the assessment of clinical psychomotor retardation. Similarly, although psychomotor slowing is an intrinsic feature of schizophrenia,
37 it should be noted that antipsychotic drug–induced bradykinesia may influence performance on psychomotor tasks.
However, when analyzing the covariation over time between the psychomotor measures, these clusters were no longer evident. Although some evidence was found indicating an association between the courses of psychomotor slowing and catatonia and between the courses of psychomotor slowing and parkinsonism, the courses of coordination and sequencing deficits were not related to each other.
A possible explanation for these contrasting findings lies within the overall stability over time of the psychomotor measures assessed: if the used outcome measures do not fluctuate over time, it seems unlikely that one would find meaningful patterns of covariation between their courses. In light of the neurodevelopmental view on psychomotor abnormalities, a prospective study in which the developmental course of different aspects of psychomotor functioning is followed might be a more appropriate design to study this question.
Nevertheless, the correlations found cross-sectionally are only modest, and the syndromic structure of the psychomotor symptom domain in schizophrenia continues to be an unresolved issue. Although psychomotor disturbances were already recognized as key features of the schizophrenic disease process by Bleuler and Kraepelin in the beginning of the 20th century, the classification of these symptoms remains problematic more than 100 years later. This should not be found surprisingly because psychomotor disturbances in psychiatry have been neglected for several decades and are still mainly approached on a phenomenological base. As a consequence, the classification and assessment of psychomotor symptoms today are mainly based on observable abnormalities in gross motor behavior. However, motor behavior is known to be the result of a complex set of subprocesses that can be impaired independently.
38 These processes have been investigated in several studies with schizophrenia patients,
39–41 but today’s diagnostic instruments fail to assess them and as such might hamper the disentanglement of the exact components of the psychomotor syndrome in schizophrenia.
Some limitations should be considered when interpreting the results of this study. First, all participants were taking antipsychotic agents, which have a known effect on psychomotor functioning. Ethical considerations hamper the conduct of a longitudinal study on unmedicated schizophrenia patients, but it should be kept in mind that the medication status of the patients may have influenced our results. Conceptual overlap and observer-based diagnostics make it very difficult to differentiate between primary psychomotor disturbances and medication-induced phenomena. Given that we studied stabilized patients that were already treated with antipsychotics, this differentiation seems even impossible. However, psychomotor abnormalities have repeatedly been shown to be intrinsic features of the schizophrenic disease process and prevalence studies on chronic antipsychotic-naïve patients indicate that psychomotor disturbances are not or only minimally heightened in patients treated with antipsychoctis compared with patients who were antipsychotic-naïve.
42 Second, control subjects were not included in this study. Therefore, we could not assess and control for normal variation over time in the psychomotor measures assessed. Especially for tasks on which it is highly likely to have learning effects, such as the CL, this hampers interpretation of the results. In this regard, one should keep in mind that the improvement over time found on the CL might be solely contributed to learning effects. Third, only half of the patients that were included entered follow-up. Although dropout seemed to be random, it does indicate that one has to be careful with generalization of the results. Last, the lack of a structured diagnostic assessment is a limitation, although all patients underwent a diagnostic interview by the main investigator and a scrutinized investigation of other sources of information such as medical files.
In sum, the results of this study are in line with a neurodevelopmental view on psychomotor functioning and could not identify any degeneration over a 1-year period in stabilized patients with schizophrenia. Although the current study in stabilized patients cannot rule out an initial improvement after treatment onset, the fact that the presence of psychomotor abnormalities remains highly frequent and stable over time indicates that the current treatment options are not sufficient to address these symptoms. Because the different psychomotor symptom clusters have been suggested to be associated with the clinical and functional outcome of schizophrenia patients,
43–45 this is an unwanted clinical outcome that deserves the attention of academia, industry, and clinicians. Academia and industry should put in more effort to disentangle the pathophysiological nature of and treatment targets for psychomotor abnormalities. Clinicians should be more aware of the psychomotor disturbances that their patients are experiencing. In psychiatric practice, psychomotor disturbances are still largely underinvestigated and underrecognized. However, the results of this study once again show that psychomotor abnormalities make part of the clinical reality of schizophrenia patients. Further research should investigate which of these psychomotor symptom clusters have the most predictive value, and additionally, which of these are sensitive to treatment. This will enhance our insight into the clinical validity of these most neglected symptom clusters of schizophrenia. Prospective studies with longer follow-up periods are needed to evaluate possible covariation in the course of different aspects of the psychomotor syndrome and to rule out the possibility of neurodegeneration in older age and/or subgroups of patients.