Several behavioral symptoms and syndromes, including impulsive, aggressive, and disruptive behaviors, conduct disorder, and attention deficit hyperactivity disorder (ADHD) are reportedly associated with disturbances in the serotonergic system. The evidence for serotonergic system abnormalities in both the peripheral as well as the CNS in children and adolescents with behavioral problems is derived from studies showing decreased 5-hydroxyindoleacetic acid (5-HIAA) concentrations in CSF, decreased [
3H]-imipramine (serotonin transporter) binding sites in platelets, and abnormal neuroendocrine responses to D,L-fenfluramine challenge.
1–3 A multitude of recent preclinical and clinical studies have revealed that early life stressors such as lack of nurturing and maternal separation as well as abuse result in persistent alterations in specific neurobiological systems.
4,5 For example, maltreated children who develop antisocial behaviors as adults have been reported to exhibit low levels of gene expression of monoamine oxidase-A (MAOA).
6,7Among adolescent males, behavioral disorders, particularly, sexual assault is one of the fastest growing crimes in the United States and accounts for >7% of all violent sex crimes. According to recent reports by law enforcement agencies, 30%‒60% of all child sexual abuse is committed by juvenile sex offenders,
8,9 2%‒4% of adolescent males over 12 years of age commit 20% of all rapes and 30%‒50% of all child molestations.
10,11 The majority of male adolescent sexual offenders exhibit emotional and behavioral problems as well as a spectrum of personality trait disturbances.
12,13 Evidence also suggests that sexually abusive youth mirror the population and generally come from all racial, economic, ethnic, and religious background.
14Serotonergic system dysfunction has repeatedly been associated with aggressive and violent behaviors in adults. There are multiple reports of decreased CSF 5-HIAAconcentrations in adults with history of aggression, poor impulse control, and violence.
17–21 Moreover, altered indices of serotonergic and noradrenergic activity in the periphery and CNS, including decreased 5-HT concentration in whole blood and platelets, decreased 5-HT uptake and [
3H]-imipramine binding sites in platelets and brain have been associated with affective disorders, hyperactivity as well as post-traumatic stress disorder among adolescents and adults.
22–24 One area for which there is paucity of evidence for neurotransmitters dysfunction, particularly that of the serotonergic system is in sexually aggressive adolescents or even adults. Our group has investigated serotonergic activity in children/adolescents with sexually offending behaviors. One adolescent male in the group who exhibited severely aggressive and sexually abusive behaviors was exposed during infancy and childhood to extreme early life stress, including physical abuse, parental drug and alcohol abuse, domestic violence, and maternal abandonment. In this report, we present our findings of a markedly abnormal profile of peripheral and central serotonergic system in this adolescent male with history of sexually aggressive behaviors.
Methods
Subject
An adolescent white male, age 15 years, with a long history of aggressive sexual offenses and disruptive behavior was referred for a psychiatric evaluation. He fulfilled the DSM-IV diagnostic criteria for conduct disorder and ADHD. His aggressive behaviors included sexual assaults against his two 14- and 16- year-old sisters, and an 11-year-old male cousin. He also had a history of destroying property, aggravated assault against older persons, including a school teacher, and a psychologist. He had a history of substance abuse, including alcohol, marijuana, and had a reported history of school suspension. He had multiple involuntary psychiatric admissions for aggressive conduct. On clinical examination, he was cooperative, surprisingly insightful, and expressed a wish to be able to control his anger. He had a normal physical examination and screening laboratory tests, thyroid function, and urine metabolic tests.
His early family life was characterized by chaos and instability. His parents had separated before he was born, and his biological mother had a history of alcoholism and mental instability. She was living with another man at the time of his birth, and she immediately abandoned him. In his childhood, he was physically abused by his biological father, who had a history of sexually aggressive and violent behaviors, and drug and alcohol abuse. His father was incarcerated for rape of his own 14-year-old daughter.
The patient was admitted to the residential treatment program at the University of Miami Miller School of Medicine/Jackson Mental Health Hospital where he remained for 15 months. He was one of the participants in a study involving a group of adolescent males with conduct disorder who were also sex offenders. All participants were admitted to the UM/Jackson residential treatment program, with no difference in the environment, and were served the same diet prepared in the residential facility. The study was approved by the Institutional Review Board of the University of Miami, and informed consent was obtained from the participants and the guardians of all participants, including our patient. However, our patient was found to be different from other participants with regards to the severity of his sexual assaults, impulsivity, and aggressiveness. He required seclusion and restraints on multiple occasions because of explosive outbursts and physical attacks on others. His disruptive behavior symptoms were partially alleviated by a combination of psychotropic medications, including treatment with carbamazapine (300–600 mg/day), thioridazine (50–100 mg/day), and imipramine (50 mg/day), and a well-designed structured behavior management program, but his sexually aggressive and offending behaviors did not improve. His treatment with psychotropic medication was discontinued, and after the required wash out period (>2 weeks), he was treated with fluoxetine (20 mg/day). Although fluoxetine transiently improved his irritability and impulsivity and he showed interest and motivation in the treatment, he continued to have intense compulsive sexual thoughts and urges, with no improvement in his sexually aggressive behaviors. He was described as “resistant and avoidant”, having great difficulty in modulating his anger, and his treatment with fluoxetine was discontinued.
The neuropsychological evaluation revealed that he had a Full Scale IQ score of 83 (WISC-III), which fell in the below average range. His verbal IQ (VIQ) was 91, and his performance IQ (PIQ) score was 78. His emotional and behavioral functioning was characterized by dysphoria and intense anger. His responses to The Robert's Apperception Test were “violent and aggressive.” He had developmental reading and expressive writing disorders (Wide Range Achievement Test, WRAT) and was diagnosed as having conduct disorder, solitary aggressive type. There was no evidence of psychosis.
After discontinuation of fluoxetine for the recommended washout period (>2 weeks), and before institution of other pharmacological interventions, we studied the patient’s serotonergic, and neuroendocrine systems.
Procedures
Assessment of serotonergic activity in our patient and other adolescent male participants (N=37) was performed after explaining the study protocol to them and to their guardian and obtaining informed consent. Because platelets are considered a peripheral model for CNS serotonergic neurons,
25 we determined platelet serotonergic function including measurement of 5-HT concentration and platelet 5-HT uptake kinetics (K
m and V
max) using platelet rich plasma (PRP), freshly prepared by low speed centrifugation of fasting blood samples collected in tubes containing acid citrate dextrose (ACD) as described earlier.
26,27 Platelet free plasma (PFP) was prepared by centrifugation of PRP at 3500 rpm. Since we found markedly reduced concentration of 5-HT in platelets of our patient, and also found abnormality in his platelet [
14C]-5-HT uptake kinetics, we assessed CNS serotonergic activity only in this patient by measuring the prolactin (PRL) and cortisol (CORT) response to D,L-fenfluramine (D,L-FEN), using the protocol described by Siever et al.
28 D,L-FEN stimulates the presynaptic release of 5-HT and inhibits its reuptake by 5-HT transporter, resulting in a net increase in the synaptic concentration of 5-HT which in turn stimulates PRL and CORT release.
29,30 An attenuated response reflects pre/and or post-synaptic 5-HT dysfunctions.
31,32Laboratory Procedures
Platelet 5-HT concentration was measured in PRP, and PFP of all participants (N=37) before treatment with any medication, including aspirin, fluoxetine, or other psychotropics. After overnight fasting, blood samples were obtained between 8 and 10 a.m. after resting for 15 minutes. PRP was prepared as described above. Aliquots of PRP and PFP were stored at –70°C until assayed for 5-HT by HPLC-ECD,
26 while a 100 µl aliquot of PRP from 17 participants, including one from our patient was used within 2 hours to measure [
14C]-5-HT uptake and kinetics, as described earlier.
27 Values of K
m and V
max were determined by Lineweaver-Burke plot.
33 Because we found deviant values of platelet 5-HT functions in the first blood sample of our patient, we obtained a second sample after 6 months while he was kept free of any psychotropic medications, including fluoxetine. The blood sample was processed for preparation of PRP and PFP, and aliquots were used for assays of 5-HT concentration, 5-HT[
14C]-5-HT uptake and kinetics, as described above.
For D, L-FEN challenge in our patient, baseline levels of hormones (PRL, CORT) were measured in two blood samples obtained in the morning at 15 minutes intervals in heparinized tubes, prior to D,L-FEN (1.0 mg/Kg) administration and five more blood samples were obtained, each at 1 hour interval (60–300 minutes) after D,L-FEN administration. All samples were kept in ice until processed for preparation of plasma, and aliquots were stored at –70°C until assayed for PRL and CORT.
Prolactin concentration in plasma was measured by the noncompetitive immune-radiometric (IRMA) assay kits obtained from Diagnostic Systems Laboratory (DSL, Webster, TX). Intra- and inter-assay % CV for PRL are 3.5% and 8%, respectively, and sensitivity is 0.1 ng/ml. Cortisol was measured by the radioimmunoassay (RIA) method using kits from Diagnostic Products Company (DPC, Los Angeles, CA). Intra-and inter-assay coefficients of variance (%CV) for CORT are 8.2% and 9.8%, respectively, and sensitivity is 0.5 µg/dl.
Statistical Analysis
Analysis of variance (ANOVA) was used to determine the mean and SD for 5-HT and hormones levels in PRP and plasma samples obtained at different time points. Student’s t test was used when applicable to examine the significance of difference in means of platelet 5-HT levels and uptake profile between our patient and the other boys of similar age in the study group.
Results
We found markedly reduced 5-HT concentration in both samples of PRP as well as PFP of our patient (obtained within a 6 months interval), compared with that in the other participants in the study who were admitted in the same residential program for treatment of conduct disorder and sex offending behaviors (
Table 1, platelet 5-HT, our patient 2.4, 3.4, mean±SD=2.90±0.7 ng/10
8 platelets, versus other participants, 121.1±47.57 ng/10
8 platelets, N=36; p<0.0001; and plasma 5-HT, our patient, 0.9 and 2.0 ng/ml, mean±SD=1.45±0.77/ml versus other participants, 2.18±1.83/ml, N=36, p <0.01 (our unpublished data) as well as plasma 5-HT levels reported in other studies 27.68±32.29/ml
66), although the number of platelets/ml blood in our patients were not different from the other participants (mean±SD=2.61±0.00 versus 2.68±0.49/ml PRP, N=36, p=NS, our unpublished data). Moreover, kinetics of 5-HT uptake in our patient’s platelets were found to be strikingly abnormal with the rate of total as well as active [
14C]-5-HT uptake exhibiting extremely low values (0.5±0.04/10
7platelets,
Table1). At
0.5 µM concentration of [
14C]-5-HT, the rate of active uptake (pmoles/minute/10
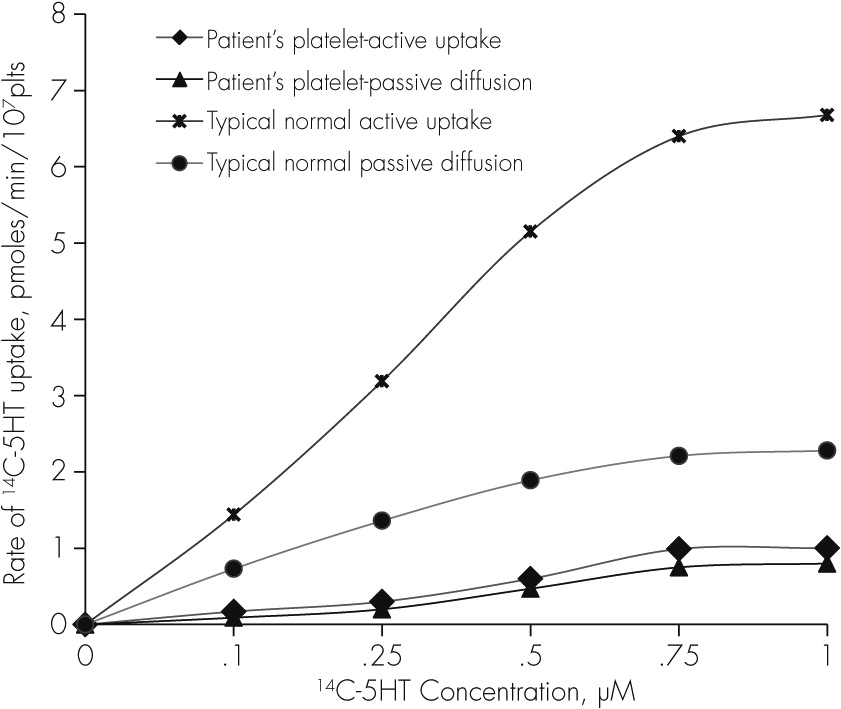
7platelets) in two samples of PRP obtained from our patient at 6 months interval, and incubated at 37°C for 4 minutes was essentially the same as passive diffusion of [
14C]−5-HT in his PRP incubated at 0°C for 4 minutes (
Figure 1); and the uptake kinetics, K
m and V
max could not be determined from the Lineweaver-Burke plot.
33 Whereas, in other boys in the study group (N=16), the rate of active uptake of [
14C]-5-HT in platelet at 0.5 µM concentration was more than 10 times higher (5.17±1.32 pmoles/min/10
7 platelets) and uptake kinetics determined by Lineweaver-Burke plot were, K
m= 1.12±0.57 µM, and V
max=15.6±5.62, N=16 (our unpublished data,
Table1). Since all participants shared the same residential facility and were served the same diet, there were no environmental and dietary differences between our patient and other adolescent males in the group that could have influenced the observed serotonergic abnormalities in our patient.
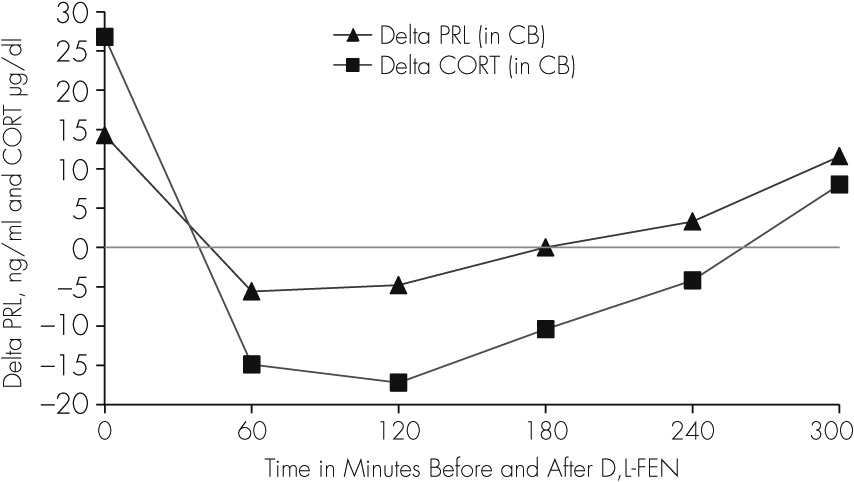
The baseline plasma concentration of both PRL and CORT, as well as the response to D, L-FEN (
Table 2) in our patient was abnormal showing higher baseline levels (two baseline PRL levels, T1=16.1, T2=12.5 ng/ml; CORT,T1=29.4, T2=24.2), and an abnormally blunted response to D,L-FEN administration, in contrast to that reported earlier in aggressive children, and normal and depressed adults.
3,28 Delta (∆) values and percent (%) change in PRL and CORT levels were determined as the difference between the average of two baseline levels of PRL (14.3 ng/ml) as well as CORT (26.8 µg/dl) and the hormone levels in each of the five samples obtained at 1-hour interval (60–300 minutes) after D, L-FEN administration. We found that at 60–180 minutes post-D,L-FEN administration, levels of both PRL and CORT decreased from the baseline, followed by a rise in PRL levels only at 240 and 300 minutes after D,L-FEN; when CORT levels continued to decrease between 60–240 minutes and increased by approximately 30% only at 300 minutes (
Table 2,
Figure 2).
Discussion
This report documents a markedly abnormal serotonergic system in an adolescent white male with a history of sexual aggression and violent behavior. Serotonergic deficits were evident by very low concentration of 5-HT in his platelet, as well as low rate of platelet [14C]-5-HT uptake, and undetectable levels of uptake kinetics, Km and Vmax, in the face of a normal platelet counts. Deficits were also observed in central serotonergic activity as demonstrated by the high baseline plasma levels of both PRL and CORT and in the clearly abnormal response to D,L-FEN administration.
Low concentrations of platelet 5-HT (2.9 ng/10
8 platelets) in our patient was an intriguing finding because such low levels have not been reported earlier in normal, depressed, or aggressive children, adolescents, or adults with behavioral problems. Moreover, other boys in the study group with CD and sex offending behavior had higher platelet 5-HT concentration (70–160 ng/10
8 platelets (121.1±47.57, N=36, our unpublished data,
Table 1). Previous studies have also reported higher 5-HT concentration (250±140 and 220±110 ng/ml PRP) in adolescent boys with conduct disorder, ADHD and aggressive and nonaggressive behaviors (
Table 1).
34 In contrast, higher 5-HT levels in whole blood were reported in children with hyperactivity and autistic disorder (228–309 ng/ml) and in adolescents <16 years old without autistic disorder (200–230 ng/ml).
35–38 However, in separate studies carried out in adults and adolescents with depression, we found lower range of platelet 5-HT concentrations (30–80 ng/10
8 platelets, and 37–65 ng/10
8 platelets, respectively)
39 compared with that in normal adults (60–150 ng/10
8 platelets).
40,41In addition, platelet [
14C]-5HT uptake mechanisms in our patient were found abnormal with values of active uptake too low for even calculation of K
m and V
max.33 The rate of active uptake of [
14C]-5-HT in this patient’s platelets incubated at 37°C remained essentially the same as passive diffusion of [
14C]-5-HT in his PRP incubated at 0°C (
Figure 1), suggesting the absence of platelet 5-HT transporter activity. In fact, the rate of active [
14C]-5-HT uptake in our patient’s platelets was even lower than that of passive diffusion of [
14C]-5-HT in platelets of a typical sample of an age matched normal adolescent male in the group with significantly higher rate of active [
14C]-5-HT uptake than the passive diffusion, as normally expected (
Figure 1). These findings suggest that our patient’s platelets were severely dysfunctional with respect to 5-HT transporter activity. However, it is unclear whether the low levels of platelet 5-HT were due to impairment in 5-HT reuptake mechanism(s) or due to a deficiency of serotonin
per se. Although our observations support the earlier findings of reduced [
3H]-imipramine binding sites on platelets of children with conduct disorder,
2 reduced density of platelet 5-HT
2A receptors in delinquent adolescents,
42 and in boys with adverse rearing and familial psychopathology of antisocial disorder,
43 to our knowledge, this report is the first to document such low levels of 5-HT in platelets (2.9 ng/10
8 platelets) and undetectable platelet [
14C]-5HT uptake kinetics (K
m and V
max) in a male adolescent with indiscriminating behaviors of sexual assaults.
Our findings of dysfunctional peripheral 5-HT system were also supported by the abnormalities we observed in the CNS 5-HT activity as inferred from the baseline levels of both PRL (14.3 ng/ml) and CORT (26.8 µg/dl), which were 2–3 times higher than the levels reported in adolescent boys having disruptive and aggressive behaviors (PRL, ng/ml=5.64±1.66, 5.70±1.4; and CORT, µg/dl=7.6±3.6) and in those with adverse rearing conditions
3,34,44 and in prepubertal aggressive boys.
45,46 Baseline levels of PRL and CORT in these studies ranged between 4 and 8 ng/ml and 8 and 14 µg/dl, respectively. Higher baseline plasma levels of both PRL and CORT suggest in part disturbance in serotonergic regulation of neuroendocrine functions.
Moreover, the PRL and CORT response to D,L-FEN challenge was blunted in our patient. A normal response to D,L-FEN administration is characterized by an increase in PRL and CORT concentrations 60 minutes post D,L-FEN administration with the response peaking at 180 or 240 minutes, with a plateau or initial fall by 300 minutes.
31,47 Blunted PRL responses to D,L-FEN have been found in adults with depression,
28 and in adolescent males with antisocial and aggressive behaviors.
44,46 In this adolescent male, there was a negative response of PRL and CORT to D, L-FEN for 240 minutes, and only a marginal increase occurred at 300 minutes (
Figure 2;
Table 2). This type of negative and delayed response profile of PRL and CORT after D,L-FEN administration has not been reported in children or adolescent boys with aggressive behaviors or in adults with depression or personality disorders.
3,28,31,44–52 This abnormal response of PRL and CORT to D,L-FEN in this patient may be caused by a reduced availability of 5-HT in the CNS. To further delineate the mechanisms associated with 5-HT deficiency and its role in behaviors of adolescent sex offenders, future investigations are warranted in a larger cohort on various indices of 5-HT functions such as availability of circulating tryptophan, genetic characteristics of enzymes and proteins involved in 5-HT synthesis (tryptophan hydroxylase, 5-hydroxytryptophan decarboxylase), 5-HT receptors and their characteristics, 5-HT transporter activity, and monoamine oxidase (MAO). Perhaps more importantly, studies with positron emission tomography (PET) with [
18F]-fluoroglucose to assess glucose metabolism or blood flow in response to fenfluramine challenge may help in evaluating dysfunctional serotonergic activity in specific brain regions (orbital frontal and cingulate cortex, raphe nuclei, and amygdala) of adolescent sex offenders, as reported in recent studies in patients with impulsive and aggressive behaviors.
53 Also, application of single photon emission computed tomography (SPECT) in human brain imaging using [
123]β-CIT (iodine-123- β –carbomethoxy-3 β-(4-iodophenyltropane) as reported in recent studies
54,55 to assesses serotonin transporters and 5-HT2 receptor binding in different brain regions would be particularly innovative in this population. These studies may reveal the reduced serotonergic functions in critical brain regions that may be playing a role in modulating aggression and sex offending behavior in adolescent males.
The reduced 5-HT functions observed in this patient may be linked to his early life abuse, neglect, and maternal abandonment. Child abuse and neglect have been implicated in the development of dysfunctional neurobiological systems,
16 and children with history of neglect, maltreatment, abuse and poor parent-child emotional bonding have been found to develop abnormal and aggressive behaviors.
52–59 Adverse childhood experiences increase the prevalence rate of major psychiatric disorders in adulthood.
60 Furthermore, among the neurobiological modifications found in a cohort of maltreated children is the low levels of monoamine oxidase-A (MAOA) gene expression; these individuals developed to adulthood with antisocial behaviors, compared with the maltreated children who had higher levels of MAOA gene expression.
6,7 These findings suggest that antisocial behavior are related to low levels of MAOA gene expression (MAOA metabolizes 5-HT and catecholamines, NE and dopamine).
Studies in nonhuman primates are concordant with the view that early childhood psychosocial environmental stressors produce changes in the development of serotonergic circuits associated with aggressive behavioral patterns, analogous to human aggression.
61,62 Experimental studies in rats also reveal that early postnatal handling or non-handling of infants by the mother, and adverse environmental conditions affect the development of different brain regions, including that of the serotonergic system and its regulatory functions in the hippocampus.
61–63 It has been suggested that 5-HT modulates aggression by inhibiting behavioral responses to environmental stimuli,
64,65 and regulates the HPA axis for adaptability and responses to various stressors.
66–68 At present, our knowledge regarding the neurobiological etiology of sexual aggression is limited and warrants further investigations in a larger cohort of children exposed to adverse childhood experiences, particularly the type of rearing environment experienced by our patient.