Participants
Eight right-handed patients between the ages of 18 and 50, who met DSM-IV-TR
7 criteria for OCD, participated in the study after being recruited from the Centre for Addiction and Mental Health in Toronto, Canada. OCD patients with lifetime comorbid diagnoses, as determined by the Structured Clinical Interview for DSM-IV Disorders,
8 were excluded. However, patients with comorbid major depressive disorder and other anxiety disorders were allowed to participate, providing OCD was the primary diagnosis. All OCD subjects must have had contamination or safety-doubting as their primary obsessions, and washing-cleaning or checking behaviors as their primary compulsions. The minimum Yale-Brown Obsessive-Compulsive Scale
9 score for enrollment was 17. Subjects taking medication were weaned off so that they were medication free for 2 weeks prior to each scan. However, patients who were being treated with fluoxetine or behavior therapy at the time of the study were excluded. Eight age-, gender-, and education-matched healthy controls with no personal or family history of psychiatric illness were recruited from the community. For the variables of age and education, patient and control subjects were matched one-to-one within 3 years (see
Table 1).
10,
11Procedures
Informed consent was obtained from all participants after procedures were fully explained. All participants completed two functional magnetic resonance imaging (fMRI) scans, which were separated by a minimum 2-week period. Participants were randomized to receive either IV citalopram or placebo during their first fMRI scan and the alternate infusion during their second scan. They were blind to the infusion order. At each scan, after two 6-minute symptom-provocation baseline (preinfusion) runs were completed, a 30-minute infusion of either placebo (250 ml of 0.9% sodium chloride) or citalopram (20 mg in 250 ml 0.9% saline) was initiated. Four additional runs were conducted, two during infusion administration (midinfusion) and two immediately after the infusion ended (postinfusion), for a total of six functional runs per scan.
Each symptom-provocation run was composed of seven picture blocks presenting either aversive scenes, neutral scenes, or scenes corresponding to common OCD symptoms (i.e., checking, washing, or hoarding). Picture blocks were grouped by category and consisted of five scenes, each presented for 3.2 seconds on a black background. Immediately following the presentation of each block, participants rated how anxious the pictures made them feel using a 7-point Likert scale and a response button box. In total, 210 color pictures were used for the provocation task (45 scenes for each of the washing, checking, neutral, and aversive picture categories, and 30 scenes for the hoarding category). Pictures were chosen from the International Affective Picture System
12 to represent the neutral and aversive blocks. Scenes depicting the OCD symptoms of washing, checking, and hoarding were obtained with a standard digital camera and from an Internet search. A psychiatrist confirmed the anxiety-arousing nature of the symptom-related pictures.
Arrow blocks separated each picture block and served as a buffer to minimize potential carry-over effects. Arrows pointing either left or right were randomly displayed on the screen, and participants were asked to indicate which way they were pointing as quickly and accurately as possible by pressing the appropriate buttons. Each arrow block lasted 20 seconds and consisted of ten white arrows on a black background.
Image Acquisition and Data Analysis
Neuroimaging (fMRI) scans were conducted at Baycrest Hospital on a 3T scanner (Siemens Medical Solutions, Erlangen, Germany). Stimulus presentation was performed using Visual Basic (Visual Studio 2005; Microsoft, Redmond, Washington) on a standard computer, which also collected subjects’ anxiety ratings. The stimuli were projected on a screen placed in the bore of the magnet and seen by the participant by means of a mirror attached to the head coil. Functional images were acquired using T2* weighted gradient echo planar imaging sequences covering 30 slices (5.00 mm thick, zero gap), aligned oblique axially, and encompassing the entire cerebral cortex (repetition time=2,000 ms, echo time=30 ms, field of view=200 mm, flip angle=70°, matrix=64×64, interleaved acquisition). A high T1 weighted anatomical scan (repetition time=2,000 ms, echo time=2.63 ms, field of view=256, slice thickness=1 mm, 160 slices) was acquired after the infusions started.
Spatial preprocessing and whole-brain image analysis of MRI images were performed using Statistical Parametric Mapping, version 8 (University College London, London, United Kingdom;
http://www.fil.ion.ucl.ac.uk/spm/software/spm8). The first three images from each run were excluded from the analyses to eliminate any T2*-equilibrium effects. To correct for subject motion, images were realigned to the mean image obtained for each participant and co-registered with their T1-weighted structural image. The T1 image was segmented using template (International Consortium for Brain Mapping) tissue probability maps for gray and white matter and cerebrospinal fluid. Parameters obtained from this step were subsequently applied to the functional and structural data during normalization into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model. Images were then smoothed with a Gaussian filter, set at 6 mm full-width-half-maximum.
Single subject data were analyzed at the first level with general linear statistical models, and the time series of the images were convolved with a canonical hemodynamic response function. Each model included high-pass filtering to remove low-frequency signal drift (period=128 seconds).
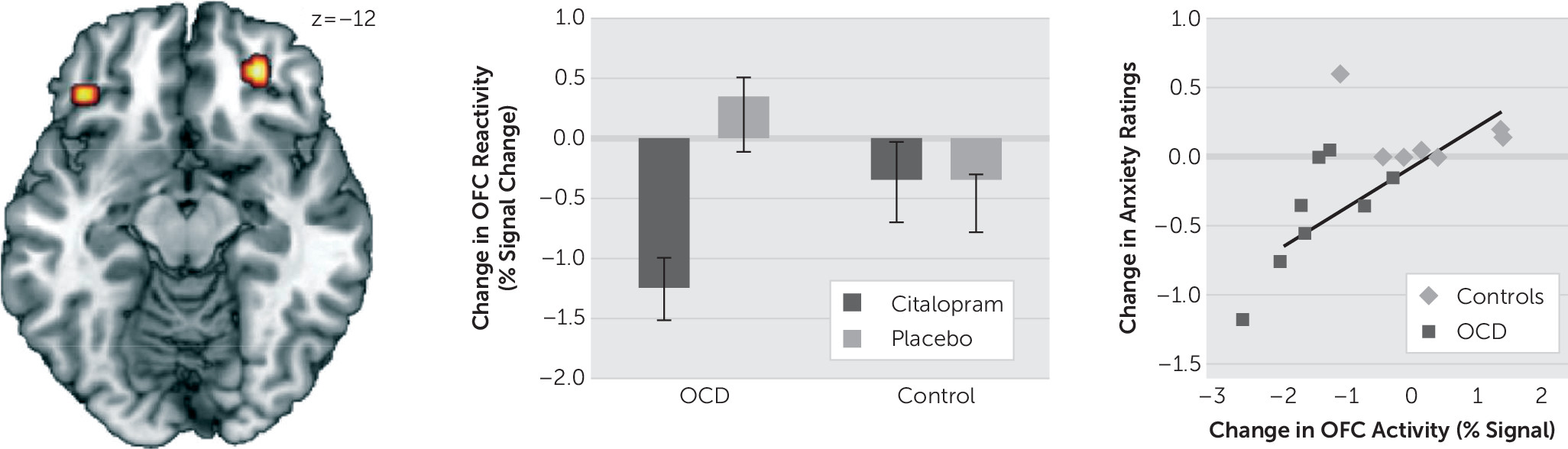
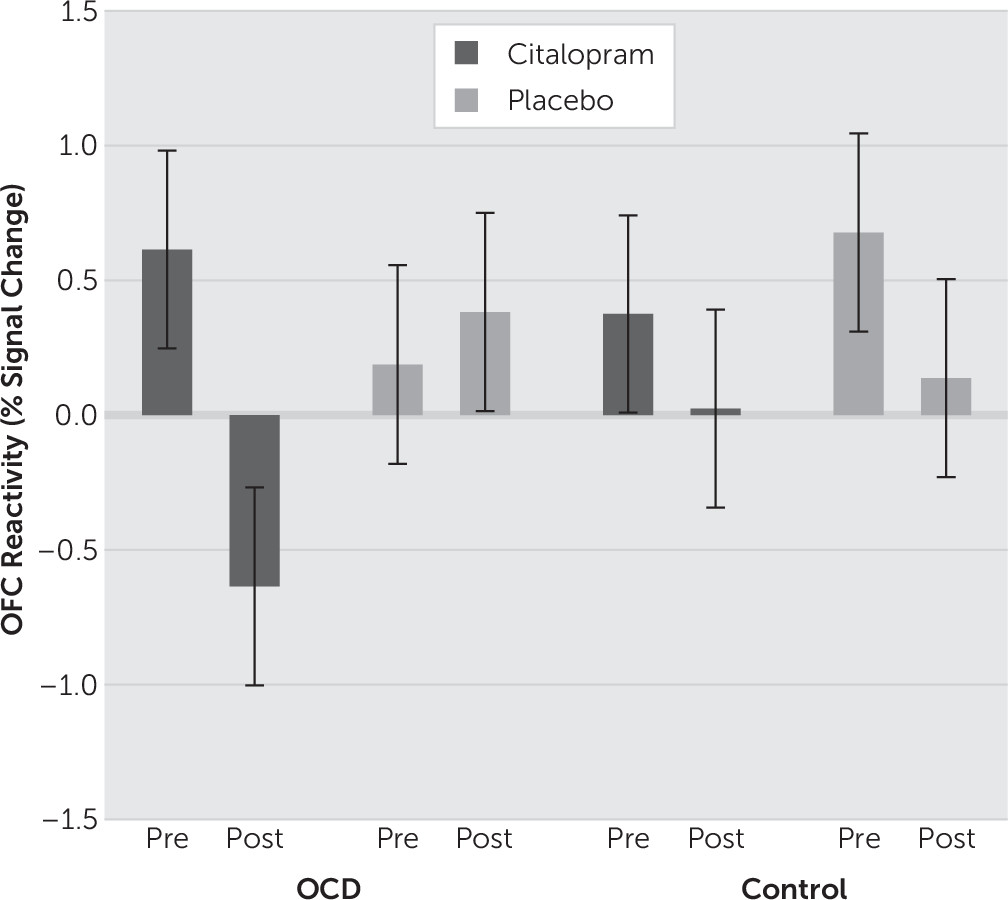
Our analysis was simplified to reflect the subtypes of the clinical sample, which featured washing and checking subtypes of OCD. Signal estimates of the washing and checking blocks were averaged together (termed “symptom” blocks) and contrasted against the neutral blocks as an index of neural reactivity. Neural reactivity was calculated for each subject at each of the pre-, mid-, and postinfusion time points. Independent samples t tests were then used to characterize reactivity differences between OCD subjects and controls at baseline. To examine the distinct effects of citalopram, mixed-model analyses of variance (ANOVAs) were performed. First, to examine initial infusion effects, a Time (Preinfusion versus Midinfusion) × Group (OCD versus Control) × Drug (Citalopram versus Placebo) ANOVA was performed. To examine full-infusion effects, a Time (Preinfusion versus Postinfusion) × Group × Drug ANOVA was performed. While the main analyses involved separately comparing the midinfusion and postinfusion scans to baseline (i.e., preinfusion scans), an additional ANOVA was conducted comparing changes in infusion-related activity across all three time points [Time (Preinfusion versus Midinfusion versus Postinfusion) × Group × Drug]. All statistical maps were set at a threshold of p<.001, uncorrected for cluster sizes greater than ten contiguous voxels, which is equivalent to a familywise error rate of P
FWE<0.05 in a Monte Carlo simulation (AlphaSim;
http://afni.nih.gov/afni/docpdf/AlphaSim.pdf).
To investigate the behavioral relevance of observed changes in neural reactivity, anxiety ratings from the checking and washing blocks were averaged to create composite symptom anxiety ratings and combined in a Group × Drug × Time ANOVA. Changes in anxiety ratings were regressed on observed changes in brain activity; correlations surviving an α<.05 Bonferroni correction were considered significant.