Cognitive impairments in PD primarily consist of slowed processing speed, multitasking/working memory difficulties, and increased forgetfulness; however, the pattern of cognitive impairment as well as the course/trajectory of cognitive decline can vary across patients.
2 For almost a decade, the concept of identifying individuals with mild cognitive impairment (MCI), who are at risk for future dementia, has been investigated as a possible meaningful concept in PD.
3 Indeed, an MCI stage has been viewed as an “intermediate” stage between cognitively intact patients with PD and those with Parkinson’s disease dementia (PDD).
2,4 Amnestic and nonamnestic MCI subtypes may also be useful in learning whether certain cognitive profiles represent an increased risk for PDD. A nonamnestic, executive dysfunction profile has been primarily associated with frontostriatal dysfunction involving the dorsolateral prefrontal cortex (DLPFC), whereas an amnestic profile may reflect a “posterior” profile with additional dysfunction in cholinergic systems and/or the beginning stages of comorbid Alzheimer’s pathology.
5,6 Evidence has been mixed regarding which MCI subtype is indicative of future PDD.
4,6,7The clinical and research utility of MCI relates to its potential to identify individuals at risk for dementia, ideally leading to early interventions that can delay the onset of dementia. However, MCI status has also been shown to relate to other meaningful outcomes. Within PD, MCI status has been linked to worse motor symptoms and stage severity, postural instability, and depression.
4,8 In normal elderly individuals, MCI status is linked not only to depression but also to apathy and anxiety.
9 Identification of mood symptoms in cognitively impaired individuals is important because such symptoms are strong predictors of quality of life that may be responsive to interventions.
9,10 Furthermore, because of a relationship between mood symptoms and cognitive functioning, treatments targeting depression, apathy, and anxiety may lead to better cognitive functioning.
11,12Apathy (disorder of motivation/goal-directed behavior), depression, and anxiety are common but dissociable mood symptoms in PD.
13 The high occurrence of mood symptoms in PD may be partly due to the reaction of being diagnosed with a serious medical condition; however, there is a direct relationship between PD pathology and emotional dysfunction (particularly apathy). Striatal dopamine depletion leads to a disruption of frontal-subcortical circuits. These circuits include cortical prefrontal regions important for emotional processing, such as the anterior cingulate cortex (ACC) and the orbitofrontal cortex (OFC).
1 In addition to dopamine depletion, high rates of mood symptoms might also be linked to dysregulation of separate neurotransmitters such as serotonin, norepinephrine, and possibly acetylcholine.
14Both mood and cognitive symptoms are important predictors of patient-centered outcomes; however, the relationship between mood disturbances and MCI status in PD has received little attention.
11,15 This study aimed to examine the relationship between MCI and common mood and motivation dysfunctions in patients with PD. The overall hypothesis is that due to similar neural circuitry (frontostriatal circuits) underlying both emotional and cognitive processes, mood symptoms will be greater among patients with PD with MCI, with apathy being particularly more severe among such patients.
Methods
Design and Participants
This cross-sectional design included a convenience sample of 214 patients with idiopathic PD according to U.K. Brain Bank criteria. The University of Florida Institutional Review Board approved this study, and consent was attained before patient participation. All patients underwent a detailed neuropsychological assessment, including completion of mood questionnaires as part of their routine clinical care through the University of Florida Center for Movement Disorders and Neurorestoration. Patients were excluded based on the following criteria: (a) previous brain surgery such as deep brain stimulation, (b) severe psychiatric disturbance (e.g., schizophrenia), or (c) severe cognitive impairment defined as a score on the Dementia Rating Scale–II below the fifth percentile.
Mood and Clinical Measures
Patients completed standard questionnaires assessing apathy, depression, and trait anxiety. Apathy was measured with the Starkstein Apathy Scale.
16 The Beck Depression Inventory–II (BDI-II) measured depressive symptoms.
17 The trait scale on the State-Trait Anxiety Inventory assessed long-standing anxiety.
18 For all measures, higher scores represent higher levels of severity for their respective construct. Data pertaining to demographics (age and education) and PD-related severity (duration of motor symptoms and ratings of motor symptoms) were collected by a movement disorder neurologist or by a trained fellow. Motor symptom severity was determined by the Unified Parkinson's Disease Rating Scale–Part III (UPDRS) motor score, which was obtained while patients were receiving medication.
19MCI Classifications
All patients completed neuropsychological measures of executive functioning, delayed verbal memory, attention/working memory, processing speed, language, and visuospatial abilities as part of a neuropsychological examination. The attention/working memory tests consisted of the forward span and backward span scores of the WAIS-III digit span subtest. Verbal memory measures included the 20-minute delay recall score from the Hopkins Verbal Learning Test–Revised and the 30-minute delayed recall of the Logical Memory Stories–II from the Wechsler Memory Scale–III. The Boston Naming Test (total correct without cues) and an Animal Fluency Test comprised the language domain. Tests of executive functioning included the Trail Making Test–Part B, the color-word interference trial of the Stroop Color-Word Test, and the letter fluency test of the Controlled Oral Word Association Test. Visuospatial tests included the Benton Facial Recognition Test and the Judgment of Line Orientation Test. Finally, the processing speed domain included the Trail Making Test–Part A, and the word reading trial of the Stroop Color-Word task. All tests were normed for age, education, and gender based on test-specific manuals or previously published norms and then converted into a z-score metric.
MCI was defined according to the Movement Disorder Society (MDS) criteria. The MDS Level II criteria were used because they are appropriate for assessments that include at least two tests within each cognitive domain: attention, executive functioning, processing speed, language, memory, and visuospatial.
12 Individuals were classified as having MCI if they performed 1.5 SDs below the mean on at least two tests. Of note, tests can be within the same domain or in separate domains. Individuals with MCI were further classified into an amnestic or nonamnestic MCI subtype. Individuals were classified into the amnestic group if they scored less than 1.5 SDs below the normed average (i.e., a z-score of less than –1.5) on either of the memory tests (Hopkins Verbal Learning Test–Revised delayed recall or Stories delayed recall).
Statistical Analyses
A series of independent t tests were computed to assess group differences. Initial analyses separated groups dichotomously (i.e., cognitively intact compared with MCI) and examined differences in apathy, anxiety, and depression. Subsequent analyses separated individuals into three groups: cognitively intact, amnestic MCI, and nonamnestic MCI. Indicators of normality were appropriate for mood variables (skewness and kurtosis values <1).
Multiple hierarchical regressions were also computed in order to covary for potential confounder variables. Mood variables (depression, apathy, and anxiety) were entered as the dependent variable for each regression. Predictor variables included MCI status, age, education, and motor symptom severity (on medication UPDRS motor subscale). MCI status was forced-entered into the model, and additional covariates (age, education, motor symptom severity) were entered in a stepwise fashion so that only predictors that significantly contributed to the model were included. Additional regression analyses were repeated to examine MCI subtypes (i.e., amnestic compared with nonamnestic). Dummy-coded MCI status variables were forced-entered into each model with additional covariates entered in a stepwise fashion.
Results
Sample Characteristics
Table 1 shows the sample characteristics of the 214 patients with PD. In brief, the sample had a mean age of 64 years and was primarily Caucasian (95.8%) and male (70.6%). Tremor was the dominant symptom in 80.8% of patients, and the average duration of symptoms was 10 years. Average UPDRS motor score while patients were “receiving medication” was 26.1. Compared with cognitively intact patients with PD, those with MCI had a longer duration of motor symptoms [t
(212)=2.27, p=0.02] and worse UPDRS motor scores [t
(212)=4.70, p<0.001]. The two groups did not differ in terms of age, education, or levodopa equivalency dose (all p values >0.05).
Almost one-half of the sample (47.2%) met criteria for MCI. Of the individuals with MCI, 59% were categorized as having amnestic MCI and 41% met criteria for nonamnestic MCI. The amnestic and nonamnestic groups did not differ in terms of age, education, UPDRS motor scores, duration of motor symptoms, or levodopa equivalency dose (all p values >0.05).
Mood Differences Among Patients With PD With and Without MCI
Independent-samples t tests were conducted to examine differences in mood scales between patients with PD with MCI and cognitively intact patients with PD. Results revealed that patients with PD with MCI reported more severe depression [t(212)=2.23, p=0.03] and trait anxiety [t(212)=2.27, p=0.02] compared with cognitively intact patients with PD. Apathy was not significantly greater in patients with MCI than in patients without MCI (p=0.05).
To control for possible confounding variables, three follow-up hierarchical regressions were conducted controlling for age, education, and severity of motor symptoms. Results revealed that the overall regression models significantly predicted depression [F(2,211)=12.80, p<0.001, r2=0.08], apathy [F(2,211)=5.60, p=0.004, r2=0.05], and trait anxiety scores [F(2,211)=5.46, p=0.02, r2=0.02]. Depressive symptoms were more severe among patients with PD with MCI (beta=0.134, p=0.02) and individuals with less education (beta=–0.237, p<0.001). Apathy symptoms were more severe among patients with PD with MCI (beta=0.129, p=0.05) and those with less education (beta=–0.172, p=0.01). Trait anxiety symptoms were more common among patients with PD with MCI (beta=0.134, p=0.02) and were not related to age, education, or motor symptom severity.
Mood Differences Among MCI Subtypes
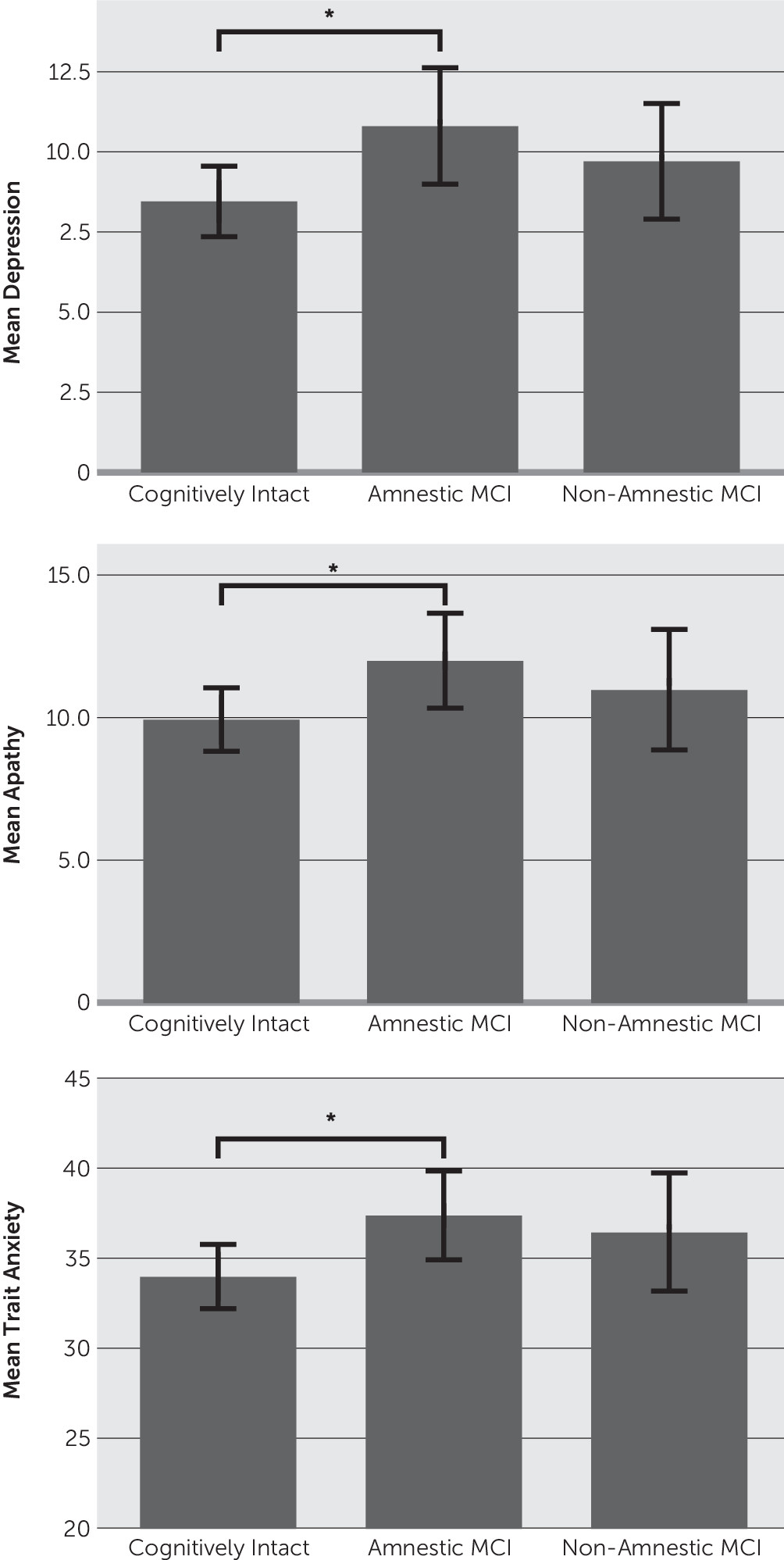
Additional analyses examined potential mood differences among amnestic, nonamnestic, and cognitively normal subtypes (
Figure 1). Results revealed that the amnestic MCI group reported significantly more severe depression, apathy, and trait anxiety compared with the cognitively intact group. The nonamnestic group did not significantly differ from either the cognitively intact or the amnestic MCI group in any mood variable (all p values >0.05).
To control for possible confounding variables, follow-up hierarchical regressions were conducted controlling for age, education, and severity of motor symptoms. The overall models significantly predicted depression [F(2,211)=9.36, p<0.001, r2=0.09], apathy [F(2,211)=4.07, p=0.01, r2=0.04], and trait anxiety [F(2,211)=3.03, p=0.05, r2=0.02]. Examination of individual predictors revealed that individuals with amnestic MCI (relative to controls; beta=0.165, p=0.01) and those with less education (beta=–0.229, p<0.001) had more severe symptoms of depression. Trait anxiety symptoms were more severe among patients with PD with amnestic MCI (beta=0.145, p=0.02) and were not significantly related to other variables. Apathy symptoms were more severe in the amnestic MCI group (beta=0.152, p=0.03) and in patients with less education (beta=–0.167, p=0.01). The nonamnestic MCI group did not significantly differ from controls on any mood measure (apathy, depression, or trait anxiety).
Correlations Between Mood and Neuropsychological Tests
Partial correlations were conducted to examine the relationship between mood (depression, apathy, and trait anxiety) and performance on neuropsychological measures. This was carried out on the entire sample, controlling for age and education (
Table 2). To minimize the number of comparisons and reduce the family-wise error rate, we computed composite scores for each neurocognitive domain: attention, verbal memory, language, visuospatial, executive function, and processing speed. We did this by converting all of the normed scores obtained for each test into z-scores and then averaging the z-scores of all tests within a single domain. Indicators of normality were appropriate for mood variables and neuropsychological composite scores (skewness and kurtosis values <1). Partial correlations revealed that higher scores on the Apathy Scale were related to worse language scores. Higher trait anxiety scores were related to worse executive function, verbal memory, and language performance. Scores on the BDI-II were not significantly related to any neurocognitive domain.
Discussion
In this study, we found that approximately 47% of our PD sample met criteria for MCI. Patients with MCI had more severe symptoms of depression and trait anxiety relative to cognitively intact patients with PD. The relationship between mood symptoms and MCI was primarily driven by the amnestic MCI group and was not accounted for by age, education, or motor symptom severity. In brief, symptoms of apathy, anxiety, and depression were greater in the amnestic MCI group relative to cognitively normal patients with PD.
These findings suggest that patients with PD with MCI may be experiencing greater mood symptoms than cognitively normal patients with PD. Identifying mood symptoms among individuals with cognitive impairment is important for at least two reasons. First, self-reported quality of life has a stronger relationship with mood symptoms (particularly depression) than cognitive symptoms.
9,10 Second, because of the relationship between mood symptoms and cognition, interventions targeting mood symptoms may have the potential to delay the progression of further cognitive impairment.
2,11 These two points are complemented by the fact that mood symptoms may be responsive to interventions, but efficacious interventions for cognitive impairment are still needed.
20To date, at least two previous studies have examined the occurrence of mood symptoms in patients with PD with and without MCI.
21,22 Both of these studies used the Neuropsychiatric Inventory (NPI), a short 12-item screener that assesses apathy, depression, and anxiety using a single item each. The smaller of these studies followed the MDS MCI classification system and found that the MCI group (N=48) differed from the cognitively intact group with PD (N=54) on the apathy item but not on the other domains or on the total NPI score.
22 In a second study with a larger sample of patients with PD (N=410), Monastero et al.
21 defined MCI subtypes (amnestic compared with nonamnestic). The major finding was greater apathy, depression, and overall neuropsychiatric symptom severity in the amnestic MCI PD group. It is important to note that Monastero et al.
21 did not fully follow the level II criteria recommended by the MDS for defining MCI. In their study, MCI criteria were based on only one test being impaired within any cognitive domain (compared with two-test impairment criteria recommended by the MDS criteria). This possibly led to a miscategorization of MCI. Taken together, discrepancies between our findings and the two previous studies likely reflect differences in how MCI was defined and operationalized and differences in how mood symptoms were assessed (i.e., a single-item measure compared with a standardized questionnaire for each mood domain).
The mechanisms underlying cognitive and mood symptoms are complicated but may overlap. Dysregulation of similar neural mechanisms may explain why mood symptoms are more common among individuals with cognitive impairment. One proposed mechanism involves the temporal-spatial pattern of dopamine degeneration in the striatum.
14 Pathological studies have shown a dorsal-ventral gradient, in which striatal dopamine degeneration begins in the dorsal-lateral head of the caudate and then spreads to the ventral-medial portions.
23 Indeed, the ventral regions of the caudate are relatively spared in the early stages of PD (compared with the dorsal regions).
24 Furthermore, although the dorsal region of the caudate projects to areas associated with cognitive functions (particularly executive functions) such as the DLPFC, the ventral regions of the caudate project to areas associated with motivational and behavioral changes such as the ACC and OFC.
1 As such, mood symptoms (particularly a loss of motivation as seen in apathy and depression) may reflect the later stage of striatal dopamine depletion in which DLPFC functioning has already been disrupted. Although this study did not find a strong relationship between mood and executive functions (traditionally associated with the DLPFC), previous studies in PD have reported relationships between executive functioning apathy and depression.
11Apathy may be particularly related to striatal dopamine degeneration; however, the mechanism for depression in PD likely includes the disruption of additional neurotransmitter systems. Indeed, past studies have shown that depression, unlike apathy, does not follow the same trajectory as motor symptoms in PD, suggesting that multiple neurotransmitters such as serotonin or norepinephrine may be involved.
25,26 Future studies are needed to elucidate the mechanism of depression, independent of apathy, in PD and to clarify the contribution of depression to cognitive profiles.
The relationship between anxiety and cognitive functioning has received scant attention in PD. Past studies of neuropsychiatric symptoms found no differences in anxiety among patients with PD who were cognitively intact, patients with PD with MCI, or patients with PDD.
21,22 These discrepancies may be partially due to the fact that these two studies assessed anxiety with a single item of the NPI rather than a full measure. Among the normal elderly population, some studies have shown trait anxiety to be associated with late-life cognitive impairment, particularly executive functioning and episodic memory, but results have been inconsistent.
27 Possible mechanisms underlying the relationship between anxiety and cognitive impairment in PD include dysfunction of the amygdala secondary to striatal dopamine depletion.
28Limitations were present in this study. First, the difference in mood symptoms among patients with and without MCI was relatively small in terms of effect sizes. Future longitudinal studies are needed to examine the directionality and temporal relationship between the development of mood symptoms and MCI as well as the clinical utility (i.e., do mood symptoms moderate the conversion from MCI subtypes to dementia?). This sample consisted of patients with PD referred for a neuropsychological evaluation from an outpatient movement disorder center. This may potentially limit the generalizability of our findings. In addition, neuropsychological measures did not include nonverbal memory tests; however, a memory impairment biased toward verbal or nonverbal information is not a common cognitive feature of PD. This study relied on self-report measures of mood, and future studies may benefit from informant/caregiver reports. Although MCI is a potentially useful diagnostic entity, issues exist regarding what tests to use, what cutoff score to use, and how many impaired tests per domain are required in defining MCI. This study utilized the MDS criteria for MCI.
2 The MDS criteria are generally consistent with
DSM-5 criteria for mild neurocognitive disorder, because both criteria require objective (both recommend 1–2 SDs below the mean) and subjective evidence of cognitive decline and relatively intact activities of daily living. However,
DSM-5 criteria do not explicitly specify the number of tests impaired, whereas MDS criteria specify impairments on at least two neuropsychological tests. In this study, 47.2% of the sample met criteria for MCI, a number that is generally consistent with previous studies in PD, which have shown 19%−53% of PD participants meeting criteria for MCI.
2 Less conservative criteria (i.e., defining participants with cognitive impairment on only one test, as opposed to two tests) would have increased the likelihood of false positive misclassification of participants.