Apathy occurs in approximately 35% of patients after stroke and is associated with more disability and lower quality of life.
1–4 Apathy is defined as a reduction in goal-directed behavior and flattening of emotions compared with the patient’s previous degree of function, not attributable to an impairment of arousal or medication side effect.
5 Apathy is typically defined using scales, based on patient report or clinician observation, all of which involve subjective interpretation.
6 As in most behavioral disorders, there is currently no objective, quantifiable definition of apathy. This serves as a limitation for the conduct of observational and clinical trial research, because subjective measures limit interrater reliability and the interpretation of outcomes (e.g., quantifying change across time).
One approach to quantify human behavior is the use of actimeters. These are small, typically wireless accelerometers or gyroscopes that can be secured to participants’ bodies to record movement, typically for many days at a time. Actimeters have been used to quantify total movement, interlimb patterns, and body position, with the potential for secondary analyses including sleep duration, circadian rhythms, and steps taken per day (reviewed by Dobkin and Dorsch
7; recent examples in stroke recovery are provided by Dorsch et al.
8 and Bailey et al.
9) Actimeters are a promising tool for clinical research because they are affordable, user-friendly, and noninvasive and they produce outputs that are ecologically valid and linearly quantifiable, unlike many standard clinical research tools.
Actimeters have already been used in studies investigating apathy to determine whether there is an association between apathy and the amount of time that participants move.
10,11 These studies were performed among patients with a variety of brain injuries, as well as among individuals with Alzheimer's disease; the results showed that apathetic patients generally move less during the day. These findings may not be extrapolated to stroke survivors, because poststroke motor dysfunction directly affects movement ability. Thus, metrics associated with quantity of movement may reflect stroke severity rather than apathy severity.
Here, we asked whether the amount of movement measured by a wristwatch-style actimeter on the unaffected wrist correlates with severity of apathy in patients with stroke. We studied patients undergoing acute rehabilitation because this setting provides a controlled environment with similar daily demands placed on all patients. This study aimed to determine the potential utility of actimeters as a measure of apathy severity and thus their potential utility in future clinical trials. We hypothesized that participants with apathy would move less throughout the day. We also performed exploratory analyses on the correlation of amount of movement with recovery during rehabilitation.
Methods
Participants
From July 2014 to May 2015, we studied patients admitted to an acute rehabilitation hospital for deficits as a result of an ischemic or hemorrhagic stroke. To ensure that the actimeter values were not affected by disorders of movement, we did not study patients with bilateral upper extremity weakness (one arm had to have a Fugl-Meyer score of 66 of 66), or movement disorders such as Parkinson's disease. We also excluded patients for which we could not diagnose apathy severity, including individuals who were taking sedating or antipsychotic medications during the day, had hypoarousal such as from an infection or sleep disturbance, or had active psychiatric disease other than depression. This study was approved by the Burke Rehabilitation Hospital Committee for Human Rights in Research, and all participants or their legally authorized representatives gave signed informed consent.
Behavioral Outcome Measures
Apathy was quantified using the Apathy Inventory–Clinician Version (AI-C),
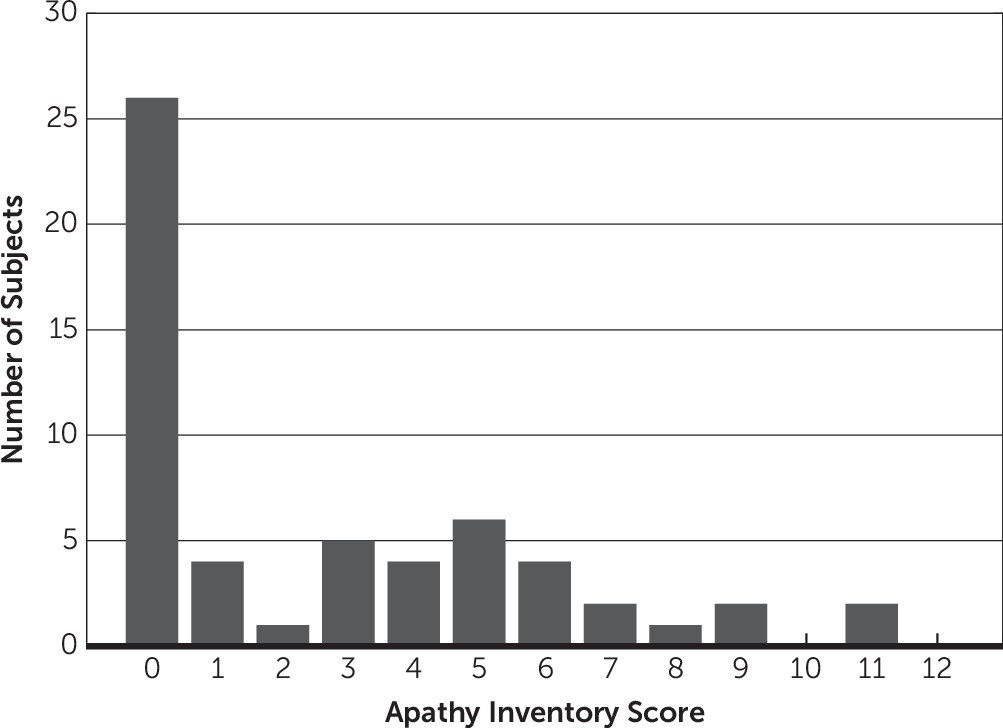
12 scored weekly by the participants’ treating speech therapist as part of standard of care. The AI-C asks the clinician to report on (a) emotional blunting, (b) loss of initiative (i.e., goal-directed behavior), and (c) loss of interest in activities and other people. Each question is scored from 0 (no problem) to 4 (major problem), with a total score ranging from 0 to 12. AI-C scores ≥4 indicate clinically significant apathy. Scores were based on behaviors observed during the previous week. We report the AI-C score closest to the time of the actimetry recording.
To quantify depressive symptoms, we interviewed patients with the Montgomery-Åsberg Depression Rating Scale (MADRS; 0–60, with higher scores representing worse depression).
13 Note that this was only performed on the subset of patients with intact communication ability (mild or no aphasia as judged by the treating speech pathologist).
Motor dysfunction was assessed with the upper extremity Fugl-Meyer motor scale by the patients’ treating occupational therapists.
14,15 This scale assesses reflexes, strength, and dexterity, with scores ranging from 0 (flaccid paralysis) to 66 (full strength). Disability was assessed at admission and discharge by treating therapists using the FIM instrument (UB Foundation Activities, Inc., Buffalo). Scores ranged from 18 to 126, with lower scores representing more disability.
For the multiple regression analysis, the Fugl-Meyer upper limb assessment was used as a measure of stroke severity. The Fugl-Meyer scale was chosen in place of the FIM because the Fugl-Meyer is an impairment measure, which means that it is less affected by other factors. For example, we have observed that apathy can directly affect many of the measures within the FIM (e.g., apathetic patients are scored lower on language measures because they do not initiate speech, although their language skills are intact).
Actimeters
Quantity of movement was assessed using a wristwatch-style actimeter (GENEActiv; Activinsights Ltd., Kimbolton, UK) that uses a triaxial accelerometer. It was set to record acceleration at 40 Hz with all data recorded onto the device in units of milli-g (1000 milli-g=1 g=9.81 m/s2). Participants wore the actimeter on the wrist of an upper extremity with intact strength (normal Fugl-Meyer score of 66). Data were collected for at least 36 hours (two nights and the intervening day), although they were only analyzed from the day and the second night. We documented the schedule of therapy sessions and meals for all patients during the day.
Actimeter Data Processing
Acceleration values were extracted from the device and were further analyzed in custom MATLAB scripts (MATLAB 2012b; MathWorks, Inc., Natick, MA) unless otherwise noted. To minimize the effect of gravity on acceleration values, we removed slowly changing acceleration values (approximately >2 seconds) using a detrending algorithm with a moving window (“locdetrend” from chronux.org
16). We then converted the three axes into one by calculating the total Euclidean distance of all axes from the origin. Finally, we binarized all acceleration values empirically into movement (>0.3 milli-
g) or no movement. This binarization removed the influence of movement amplitude from the final analyses because we were interested in the amount of time spent moving rather than its amplitude. Results are all displayed as “total movement time,” meaning the amount of time where the acceleration value signified that the subject was moving.
Statistical Analyses
Statistical analyses were also performed in MATLAB using built-in and Statistics Toolbox codes. Scatterplots were produced with the code “scatter.m” after confirming that there were no overlapping data points. Because some data were not Gaussian distributed, we report medians and interquartile ranges and compare groups using the Wilcoxon rank-sum test. Box plots were created using MATLAB’s “boxplot.m” with default parameters. Multiple linear regression analysis was performed using MATLAB’s “linearmodel.fit” with default parameters.
Discussion
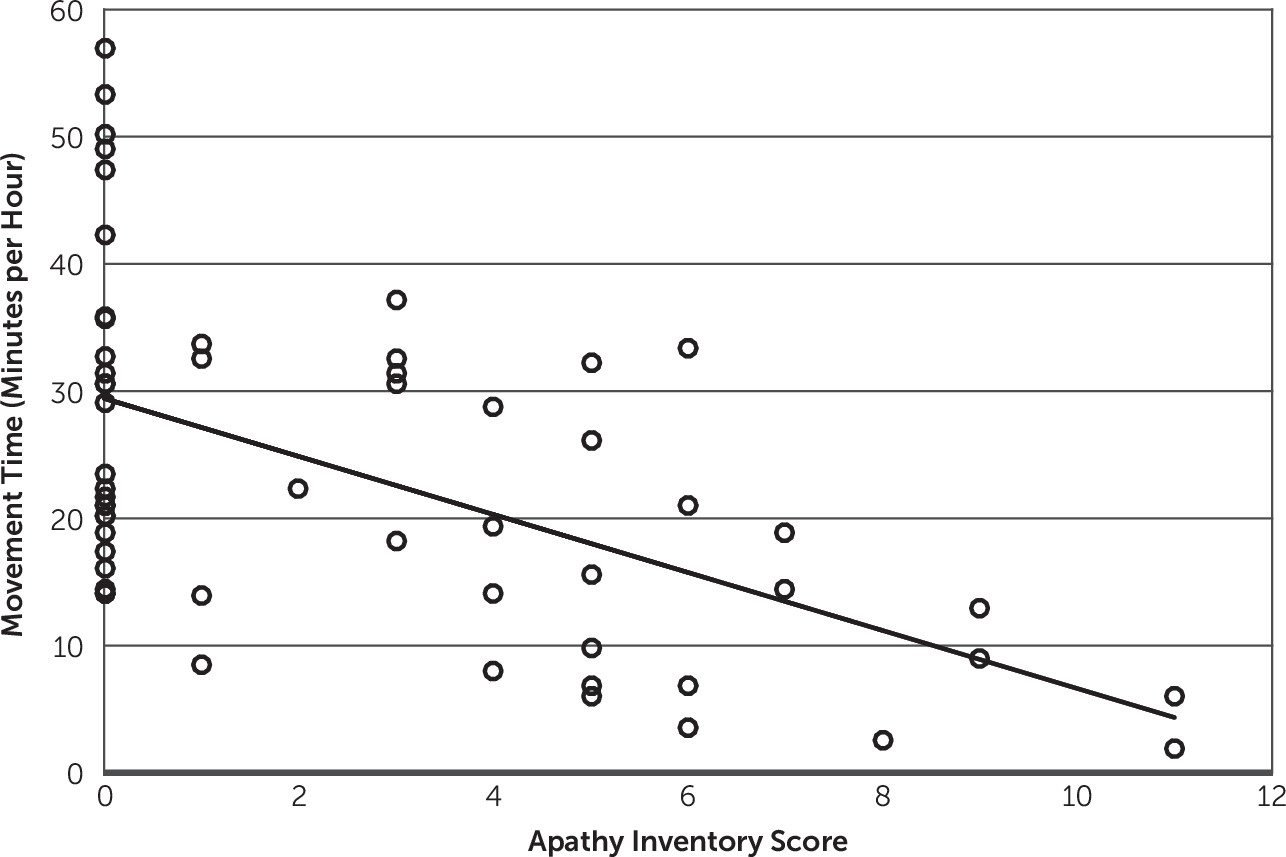
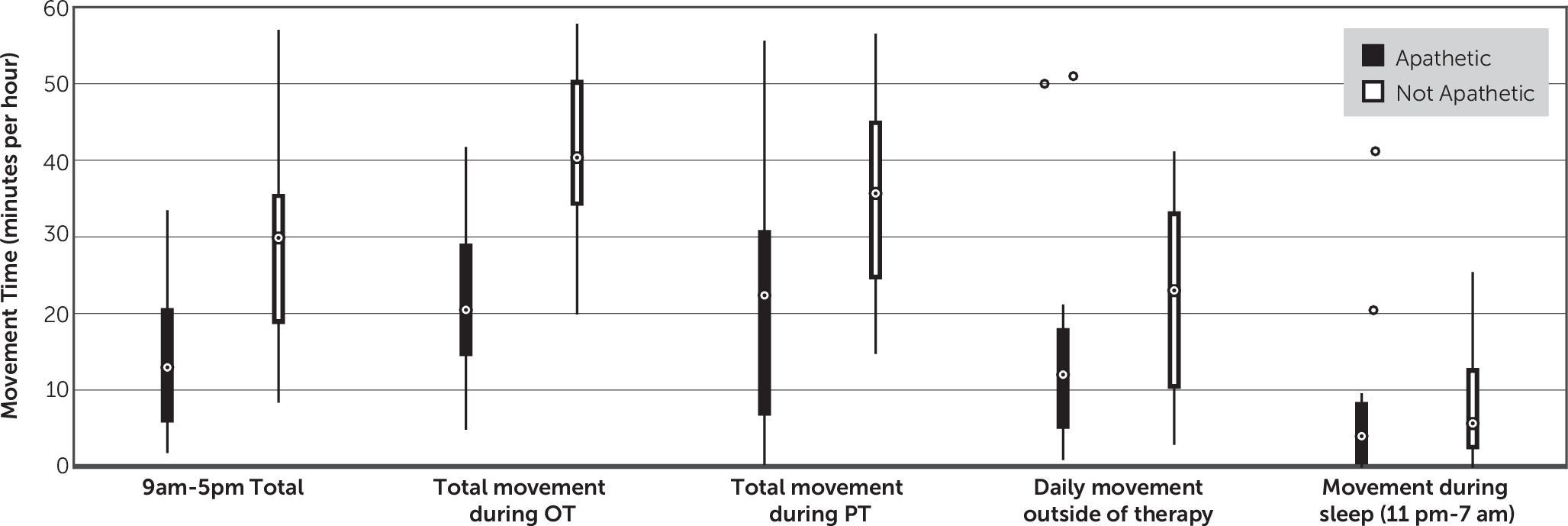
The primary finding of this study is that the amount of movement of the unaffected upper extremity among patients with stroke undergoing inpatient rehabilitation correlates well with their severity of apathy. This finding remained positive even after accounting for the severity of stroke (defined by the weakness of the patients’ more affected side) and age. These findings suggest that actimeters may serve as a useful adjunctive measurement of apathy severity.
Other studies have looked at the correlation of apathy with the amount of movement by actimeters and produced similar findings to ours. The closest study to ours was by Müller et al.,
11 who used wristwatch-style actimeters to study 24 patients with a variety of brain injuries (stroke, hypoxia, tumor, and trauma) and 12 age-matched controls. One-half of their patients had significant apathy and one-half had minimal to no apathy. The authors found that patients with significant apathy moved less, whereas patients without significant apathy were similar to controls. In addition, David et al.
10 studied 107 patients with Alzheimer's disease for 7 days and found that the 43 patients with apathy had reduced daytime mean motor activity relative to the 64 patients without apathy. These studies, combined with ours, suggest that actimeters may be useful adjunct measures of apathy in a wide range of conditions, including psychiatric diseases such as major depressive disorder and schizophrenia and other neurodegenerative conditions such as Parkinson's disease.
Both Müller et al.
11 and David et al.
10 also reported that patients with apathy had more periods of daytime napping. We did not specifically measure this because it is impossible to differentiate sleeping versus sitting still while awake with a wristwatch actimeter alone. David et al.
10 also sought to determine whether actimeters can be used to diagnose apathy. They used receiver-operating characteristic curve analysis and reported that amount of movement could be used to distinguish apathy from nonapathy, although with a sensitivity of 81% and specificity of 71%. Our findings suggest that actimeters alone cannot be used to diagnose apathy because there is significant variability in the amount of movement in patients with no signs of apathy (AI-C=0;
Figure 2); instead, actimeters could be used as an adjunctive tool in combination with other clinical assessments.
The consistent findings of inverse correlations between apathy severity and amount of daytime movement across this and other studies suggest that actimeters may be useful tools for generating outcome measures in clinical trials for treatment of poststroke apathy. As reviewed by Dobkin and Dorsch,
7 actimeters and similar noninvasive, mobile tools offer significant advantages for clinical research. These tools can record data from patients in their homes, making the information more ecologically valid and less costly. They can also record information for many days to ensure that more aspects of behavior are captured. In addition, the data these tools produce are linear and therefore easier for analysis than typical clinical scales with arbitrary scoring systems. On the other hand, current outputs from these devices are limited in that what they report may not reflect the behavior of interest (e.g., measuring all movement rather than goal-directed movement); however, advancements in algorithm and device development suggest that this hurdle can be overcome.
8,9,19A secondary finding from this study involves the role of apathy and recovery. Our finding that apathy correlates with less recovery of disability (FIM) during rehabilitation stay is consistent with other studies.
2,3,17,18 The mechanism by which apathy affects recovery is not clear. It could be due to reduced participation in rehabilitation; however, a study that looked specifically at participation found no correlation with apathy or depression.
20 The role of apathy in recovery could also be attributable to reduced practice outside of therapy—we did not measure this specifically, but we found no correlation between overall activity (movement of the unaffected extremity) and recovery among the nonapathetic patients. Previous meta-analyses have reported that amount of therapy time provided correlates with recovery after stroke.
21,22 Future studies could use actimeters on the affected extremities to more specifically determine the role of practicing of movement and rate of recovery.
This study had multiple limitations affecting interpretation and generalization. One was that our measure of apathy severity (AI-C score) may not always represent poststroke apathy. In some cases, this is because patients may have had apathy before stroke onset (families were not interviewed as part of the scoring process). In other cases, the apathy may be attributable to concurrent depression or neurodegenerative disease. These limitations are not significant because the goal of this study was to determine whether apathy could be quantified by actimeters in patients with stroke, regardless of the cause. Another limitation was that actimeters measure any movement of the extremity and are unable to specifically identify goal-directed movement, which is a core aspect of apathy. Furthermore, goal-directed behavior is only one aspect of apathy, and actimeters cannot measure goal-directed cognition and emotional expression. Finally, our statistical model of apathy severity, stroke severity, and age (
Table 2) explained less than one-half of the variance in amount of movement (adjusted R
2=0.34), suggesting that there are other unexplained factors. This remaining variability in movement time suggests that further information is required before using movement time as a diagnostic test of poststroke apathy.
The findings here suggest multiple directions of future research. One is to better explain the variability in movement time not explained by age, apathy severity, and stroke severity. This could include measures of fatigue and sleepiness, pain, or other measures of motivation. Further data should also come from inpatient and outpatient populations for longer periods to determine whether movement amount is stable and if it correlates with the change in apathy severity that occurs in some patients naturally across time.
3,23 Actimeters could be used as low-cost exploratory outcome measures in clinical trials of apathy treatments in a variety of conditions. Finally, quantification of apathy may be improved by algorithms that allow actimeters to measure goal-directed behavior, as well as the addition of other devices that can be used to measure goal-directed cognition and emotional expression.
24