Participants
Participants were recruited from the partial hospitalization program at McLean Hospital after completion of a 3- to 4-day inpatient detoxification program. Exclusion criteria included neurologic illness or injury, MRI contraindications, any psychiatric condition that would interfere with provision of consent or valid self-report, acute intoxication, substance withdrawal, pregnancy, or safety concerns. The McLean Hospital Institutional Review Board approved this study. Informed consent was obtained from participants after the study procedures had been fully explained.
Data Collection and Analyses
Substance use data in the past 30 days were collected using the Addiction Severity Index (ASI). The ASI was modified to include the question, “How many days has it been since you used [substance].” Data regarding DSM-IV substance use and co-occurring disorders were collected through the Structured Clinical Interview.
MRI scans were conducted using a Siemens Trio 3T scanner and 32-channel head coil. Structural MRI images were acquired for co-registration with the functional data using the following parameters: resolution, 1.0×1.0×1.33 mm; TR, 2.1 seconds; TE, 3.3 milliseconds; slices, 128; matrix, 256×256; and flip angle, 7 degrees. Six-minute, eyes-open, resting-state gradient echo-planar functional magnetic resonance imaging (fMRI) images were acquired with the following parameters: TR, 2.5 seconds; TE, 30 milliseconds; flip angle, 90 degrees; slices, 42; and voxel size, 3.5 mm isotropic.
All data analyses were performed using the fMRI Software Library (FSL). Motion correction, brain extraction, slice timing correction, spatial smoothing with a Gaussian kernel of full width at the half maximum (6 mm), a high-pass temporal filter with Gaussian-weighted least-squares, and straight-line fitting (100 seconds) preprocessing steps were conducted. Subject-specific data were registered to the Montreal Neurological Institute MNI-152 standard space template at 2 mm3. fMRI data were transformed using 2×2×2-mm resolution. The FSL MELODIC was used to remove sources of noise for each individual’s data.
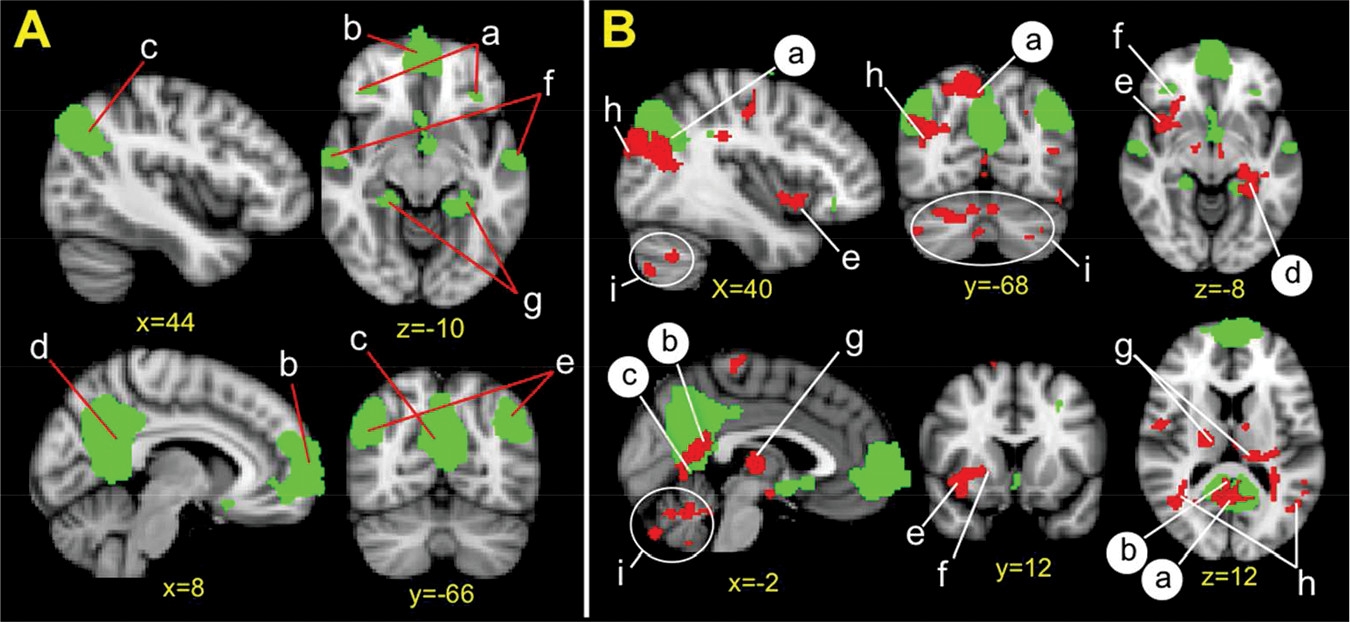
A group-level independent component analysis using FSL MELODIC was then performed to define the DMN for the participant sample. Consistent with our previous work,
11,12 dimensionality was fixed to 35 components to examine large-scale resting-state networks. The DMN was identified visually, and its spatial distribution matched previous work.
13 To calculate participant-specific time courses and spatial maps for the DMN, we used the dual regression approach implemented with FSL.
To assess whether DMN coupling varies relative to abstinence duration, we used the nonparametric permutation method (FSL Randomize) to correlate days since last substance use with DMN coupling (5,000 permutations). Cluster-based thresholding was corrected for multiple comparisons by using the null distribution of the max cluster size across the image (cluster-corrected z=2.3, p<0.05).