Effect of High-Frequency Transcranial Magnetic Stimulation on Craving in Substance Use Disorder: A Meta-Analysis
Abstract
Methods
Development and Registration of Protocol
Search Strategy
Study Selection Criteria
Types of studies.
Types of participants.
Types of interventions.
Types of outcome measures.
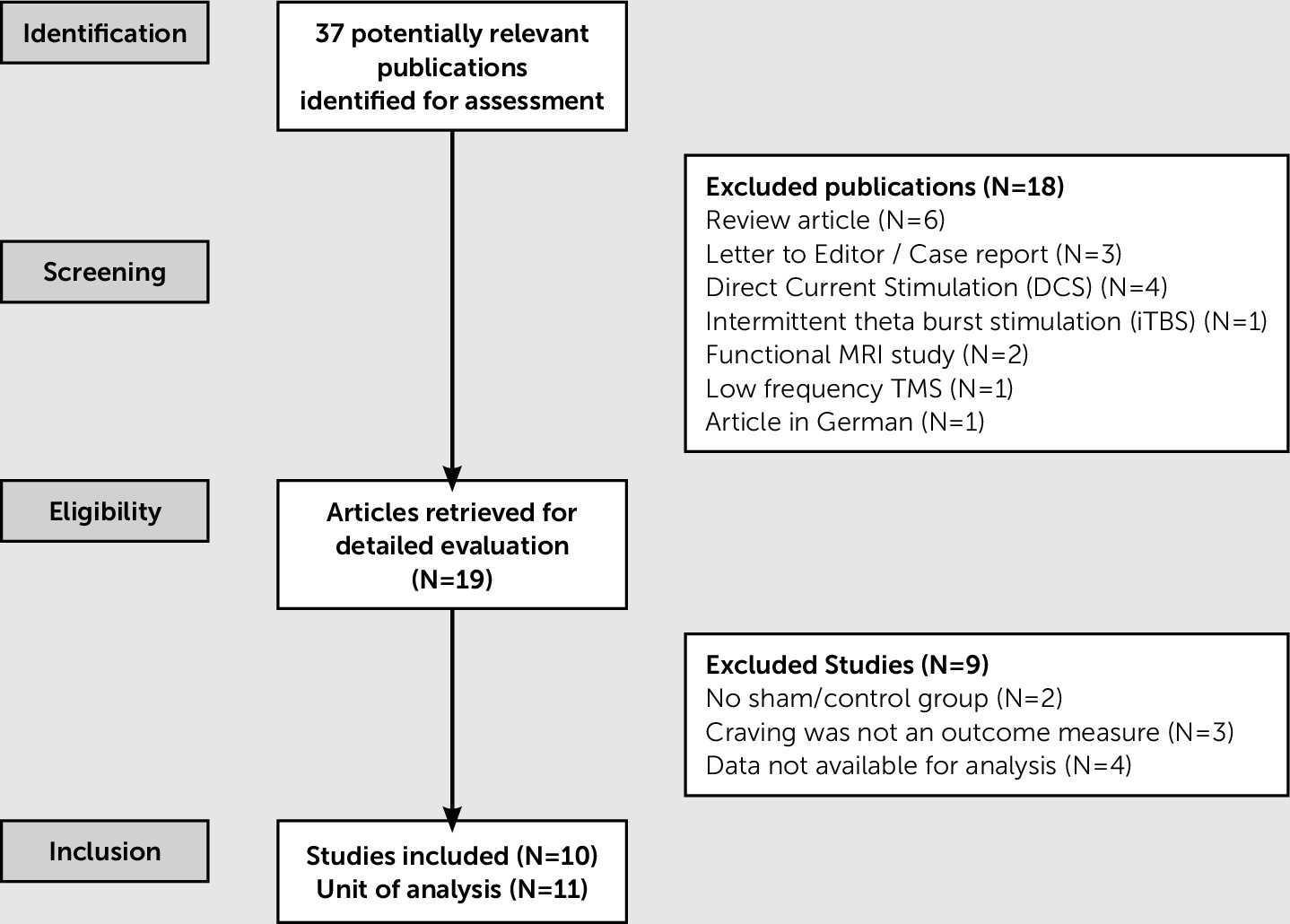
Study Selection and Data Collection
Selection of studies.
Data extraction and management.
Data Analysis
Assessment of risk of bias in included studies.
Measures of treatment effect.
Unit of analysis issues.
Assessment of heterogeneity.
Subgroup and sensitivity analysis.
Assessment of publication bias.
Results
Description of Included Studies
| Trial and Location | Methods | Participants | Interventions | Outcomes | Number of Participants | Site of Stimulation | Intervention Protocol | Notes/Remarks |
|---|---|---|---|---|---|---|---|---|
| Amiaz et al.11; Israel | Randomized, double-blind, sham-controlled | Patients with nicotine use disorder | High-frequency rTMS | VAS and sTCQ | Sham smoke group: 9/11 subjects finished the 10 days of treatment Real smoke group: 12/13 subjects finished the 10 days of treatment | Left DLPFC | 10 Hz-rTMS, 100% MT, 20 trains/day with an intertrain interval of 15 seconds | Significant reduction in craving |
| Mishra et al.18; India | Randomized, single-blind, sham-controlled | Right-handed male patients with alcohol use disorder | High-frequency rTMS | ACQ-NOW | Sham rTMS: 15 patients; Active rTMS: 30 patients | Right DLPFC | 10 Hz-rTMS, 4.9 seconds per train, intertrain interval 30 seconds, 20 trains per session, total 10 sessions, 110% MT | Significant reduction in craving |
| Höppner et al.21; Germany | Randomized, sham-controlled | Female patients with alcohol use disorder | High-frequency rTMS | OCDS | Sham rTMS: 9 patients Active rTMS: 10 patients | Left DLPFC | 20-Hz, 20 trains of 2.5 seconds, intertrain interval of 42.5 seconds, 1,000 stimuli per day on 10 consecutive working days, 90% MT | No significant changes in craving |
| Herremans et al.22; Belgium | Randomized, single-blind sham-controlled, crossover design | Patients with alcohol use disorder | High-frequency rTMS | OCDS | Sham rTMS: 16 patients; Active rTMS: 15 patients | Right DLPFC | 20-Hz, 40 trains of 1.9 seconds, intertrain interval of 12 seconds, 1560 pulses per session, 110% MT | No significant changes in craving |
| Herremans et al.23; Belgium | Randomized, single-blind sham-controlled, crossover design | Patients with alcohol use disorder | High-frequency rTMS | OCDS | Sham rTMS: 16 patients; Active rTMS: 13 patients | Right DLPFC | 20-Hz, 40 trains of 1.9 seconds, intertrain interval of 12 seconds, 1,560 pulses per session, 110% MT | No significant changes in craving |
| Li et al.12; United States | Randomized, double-blind sham-controlled, crossover design | Right-handed patients with nicotine use disorder | High-frequency rTMS | VAS | 16 (14 completed the study) | Left DLPFC | 10-Hz for 5 seconds, intertrain interval of 10 seconds, 100% MT, each session of 15 minutes with 3,000 pulses | Significant reduction in craving |
| Dinur-Klein et al.33; Israel | Randomized, double-blind sham-controlled | Patients with nicotine use disorder | High- and low-frequency deep rTMS | sTCQ | Sham group: 31 subjects; Low frequency group: 14 subjects; High-frequency group: 32 subjects | Insula and the lateral prefrontal cortex bilaterally | High-frequency sessions consisted of 33 trains of 10 Hz, each lasting 3 seconds, with an intertrain interval of 20 seconds. Total treatment duration was 760 seconds with 990 pulses. Low-frequency sessions consisted of 600 continuous pulses at 1 Hz. | No significant change in craving |
| Pripfl et al.13; Austria | Sham-controlled within-subject design | Patients with nicotine use disorder | High-frequency rTMS | A 5-point rating scale following Cue induced craving (CIC) task | 14 (11 analyzed) | Left DLPFC | 10 Hz, 24 trains, 5 seconds per train, 25 seconds intertrain interval, i.e., 1,200 pulses within 11.6 minutes, 90% MT | Craving ratings were significantly lower after real rTMS stimulation compared with sham stimulation |
| Girardi et al.19; Italy | Open-label add-on deep TMS compared with standard treatment | Patients with alcohol use disorder | High-frequency deep TNS | OCDS | Add-on deep TMS group: 10 subjects; Standard drug treatment group: 10 subjects | Bilateral DLPFC | 20 Hz, 55 trains per session with a 2-second duration each and an intertrain interval of 20 seconds, 120% MT, total of 20 sessions for each patients | Add-on of deep TMS to standard treatment is associated with a significant reduction in craving |
| Ceccanti et al.20; Italy | Randomized, double-blind placebo-controlled | Patients with alcohol use disorder | High-frequency deep rTMS | VAS | Real stimulation: 9 subjects; Sham stimulation: 9 subjects | Medial PFC | 20 Hz, 10 sessions of 30 consecutive trains of 50 stimuli, intertrain interval of 30 seconds, 120% MT | Significant reduction in craving after real rTMS stimulation |
| Trial and Location | Methods | Participants | Intervention | Outcomes | Number of Participants | Site of Stimulation | Intervention Protocol | Reason for Exclusion |
|---|---|---|---|---|---|---|---|---|
| Eichhammer et al.14; Germany | Double-blind crossover trial | Treatment-seeking subjects with nicotine use disorder | High-frequency rTMS | Number of cigarettes smoked during an ad libitum smoking period and effects on craving after a period of acute abstinence | 14 | left DLPFC | 20 trains of rTMS at a rate of 20 Hz for 2.5 seconds over 14 minutes (1,000 pulses/session; intertrain interval: 42.5 seconds). | Published data are inadequate to calculate standardized mean difference. Request mail and subsequent reminders were sent to corresponding author for data with no reply. |
| Camprodon et al.16; United States | Randomized crossover study | Right-handed males with cocaine use disorder | High-frequency rTMS | VAS | 6 | Right and left DLPFC | Each session consisted of 20 trains of a 10-second duration, separated by 1-minute pauses. The frequency of stimulation was 10 Hz, and the intensity was 90% of the individual’s motor threshold. | No placebo/sham comparator group. |
| Staroverov et al.30; Russia | Randomized, placebo-controlled study | Patients with stage II alcohol use disorder | TMT | Hospital Anxiety and Depression Scales, Zung Scale, Spielberger-Khanin Scale, MFI-20 Questionnaire, functional disorders of the autonomic nervous system | Total: 62 | Running magnetic field was directed bitemporally by moving the field from the temporal lobes to the occipital area. | The field scanning frequency was 1–12 Hz with a smooth increase within this range from one procedure to the next during the course of treatment. Courses consisted of 10 daily procedures with exposures lasting 10–20 minutes. | No mention of method of assessment of craving. |
| Study group: 32; Control group: 30 | The intervention protocol has not been discussed in detail. | |||||||
| E-mail address of the corresponding author has not been provided in the translated article (author could not be contacted). | ||||||||
| Staroverov et al.38; Russia | Randomized add-on study | Patients with alcohol use disorder | Transcranial dynamic magnetotherapy | Severity of anxiety and depression, memory, attention, function of autonomic nervous system, psychoemotional status | Total: 54 | Directed bitemporally by moving the field from the temporal lobes to the occipital area. | 1–12 Hz with a smooth increase within this range from one procedure to the next during the course of treatment. Courses consisted of 10 daily procedures with exposures lasting 10–20 minutes. | Craving was not an outcome measure. |
| Study group: 29; Control group: 25 | ||||||||
| Rose et al.15; United States | Single-arm repeated-measures design | Volunteer subjects with nicotine use disorder | High- and low-frequency rTMS | Shiffman-Jarvik Questionnaire, Cigarette Evaluation Questionnaire | 15 | Superior frontal gyrus (SFG), Motor cortex (control condition) | Stimulation was carried out for all three conditions (10-Hz SFG, 1-Hz SFG, 1-Hz MOC) for three periods of 2 minutes and 30 seconds in duration (total period of stimulation 7.5 minutes) at an intensity that was 90% of MT. Thus, the number of pulses was equal for the two 1-Hz conditions, but a greater number of pulses was administered during 10-Hz stimulation. | Published data are inadequate to calculate standardized mean difference. Request mail and subsequent reminders were sent to corresponding author for data with no reply. |
| Prikryl et al39; Czech Republic | Double-blind randomized, placebo-controlled study | Male schizophrenia patients with nicotine use disorder | High-frequency rTMS | Number of cigarettes smoked | Total: 35 | Left DLPFC | Intensity of magnetic stimulation in % of motor threshold (110%), stimulation frequency (10 Hz), number of trains, single train duration (10 seconds), intertrain interval (30 seconds), and total number of stimulation sessions. In each stimulation session, 2,000 TMS pulses were given, with a total of 42,000 pulses per treatment course. | Craving was not an outcome measure. |
| Active stimulation: 18; Sham (placebo): 17 | ||||||||
| Vicario et al.40; Italy | Nonrandomized placebo-controlled | Subjects with nicotine use disorder | Low-frequency TMS | Motor-evoked potential amplitudes and resting motor thresholds recorded from of both tongue and extensor carpi radialis | Total: 20 | Corticobulbar area | TMS frequency during the experimental blocks was less than 0.1 Hz, intensity of 120% of resting motor thresholds. The magnetic stimulation was delivered randomly between 1,000 and 1,300 ms from stimulus onset. | Craving was not an outcome measure. |
| Test group: 11 | ||||||||
| Placebo group: 9 | ||||||||
| Mishra et al.29; India | Randomized, double-blind, active comparator study | Right-handed male patients with a diagnosis of alcohol use disorder | High-frequency rTMS | Severity of Alcohol Dependence Questionnaire Form-C | Total: 20 | Right or left DLPFC | At 110% of the MT determined. | No placebo/sham comparator group. |
| and ACQ-NOW | Right DLPFC: 10, left DLPFC: 10 | High-frequency (10 Hz) stimulation was administered for 4.9 seconds per train, with intertrain interval of 30 seconds, and a total of 20 trains per session. Each patient received 1,000 pulses per day. | ||||||
| Terraneo et al.17; Italy | Between-subject open-label randomized, clinical trial | Treatment-seeking patients with cocaine use disorder | High-frequency rTMS | Cocaine use during the last month (frequency and daily amount), cocaine craving score | Total: 32 | Left DLPFC | 15-Hz frequency, pulse intensity 100% of the resting motor threshold, 60 pulses per train, inter train pause of 15 seconds, 40 stimulation trains, and 2,400 total pulses for a total duration of 13 minutes. | Published data are inadequate to calculate standardized mean difference. Request e-mail and subsequent reminders were sent to corresponding author for data with no reply. |
| Test group: 16; control group (standard treatment): 16 |
| Included Studies | Risk of Bias | ||||||
|---|---|---|---|---|---|---|---|
| B1a | B2b | B3c | B4d | B5e | B6f | B7g | |
| Amiaz et al.11 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Mishra et al.18 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Höppneret al.21 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Herremans et al.22 | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Herremans et al.23 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Li et al.12 | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk |
| Pripfl et al.13 | Low risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Dinur-Klein et al.33 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Girardi et al.19 | Unclear risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Low risk |
| Ceccanti et al.20 | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk |
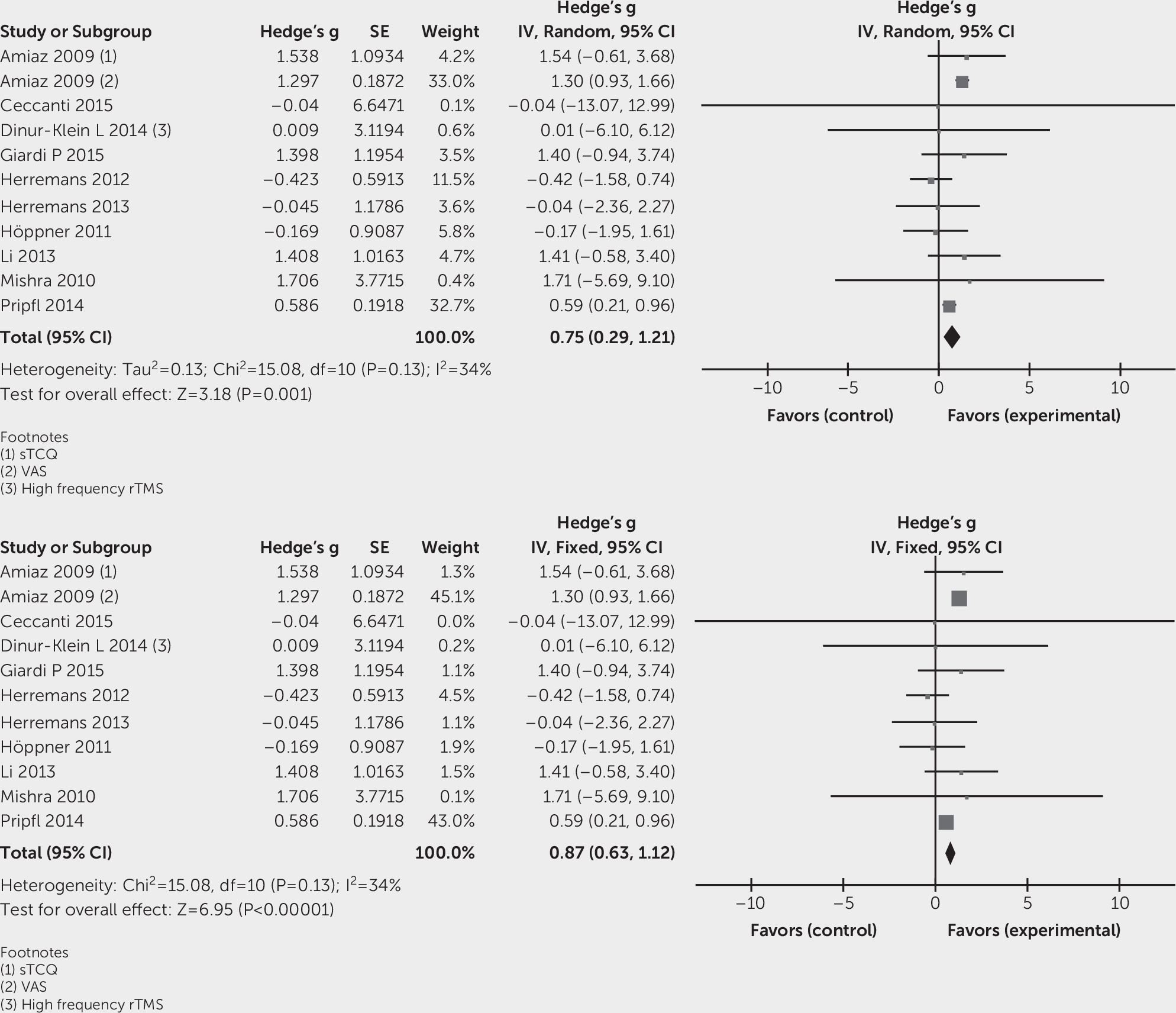
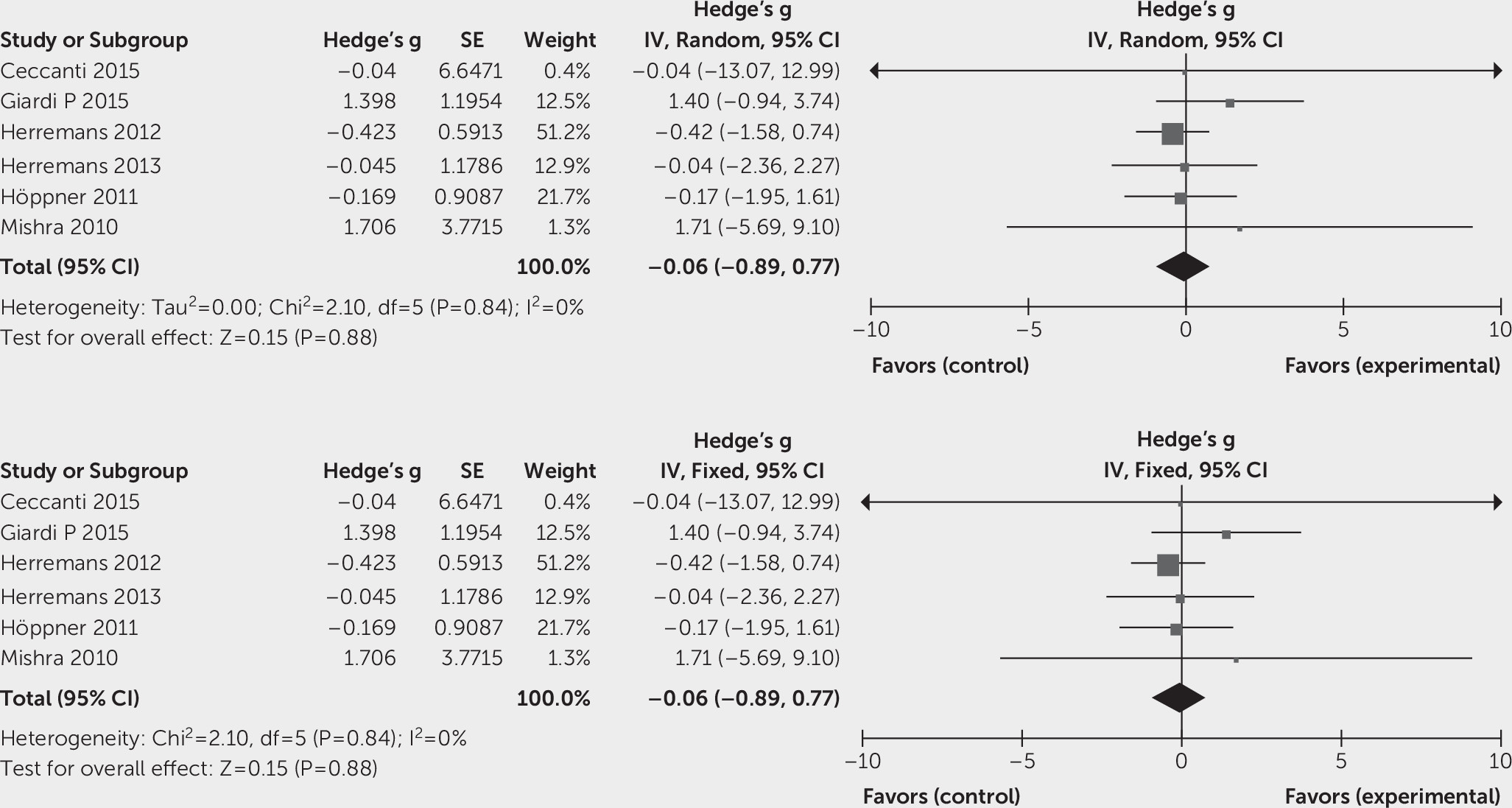
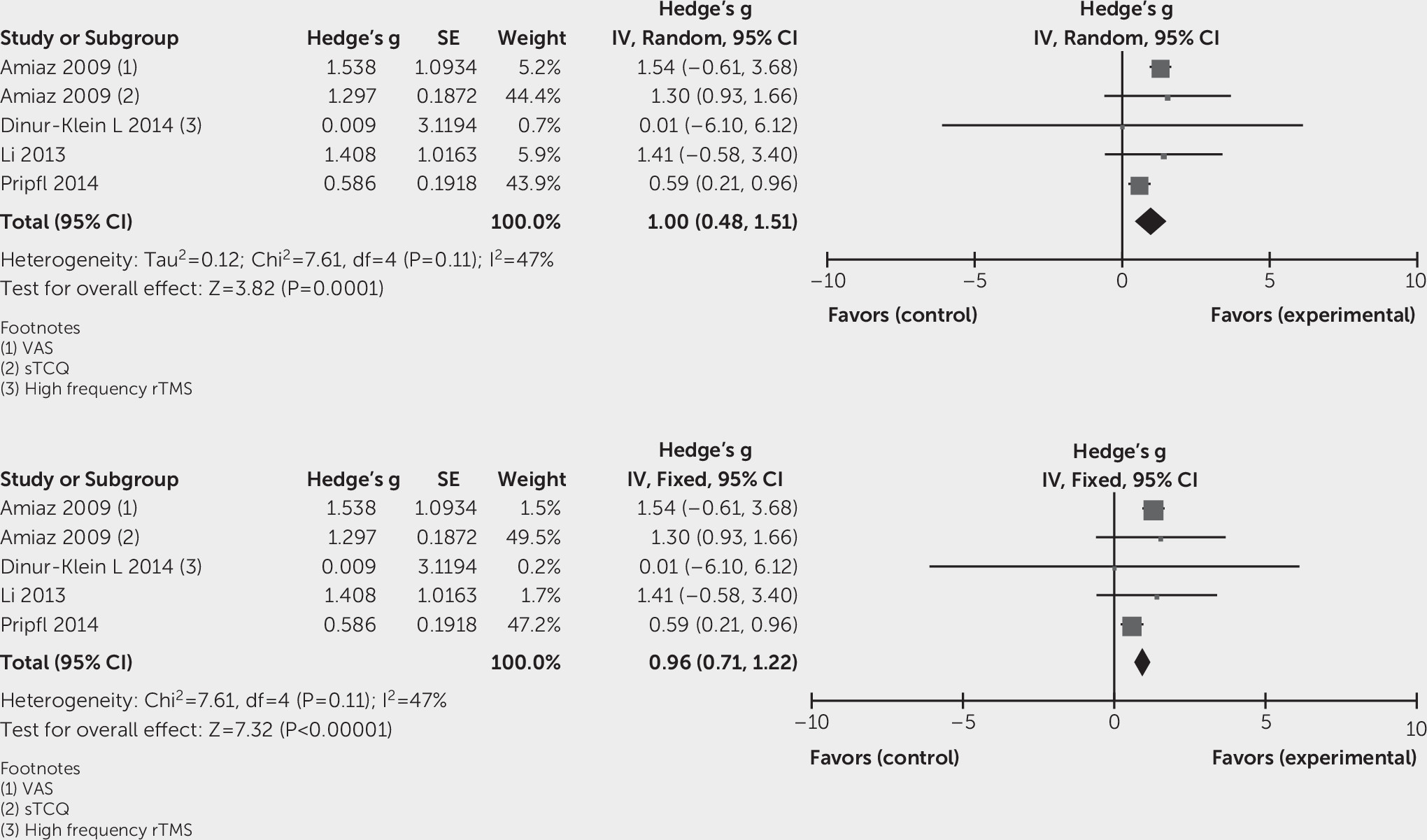
Effects of Intervention
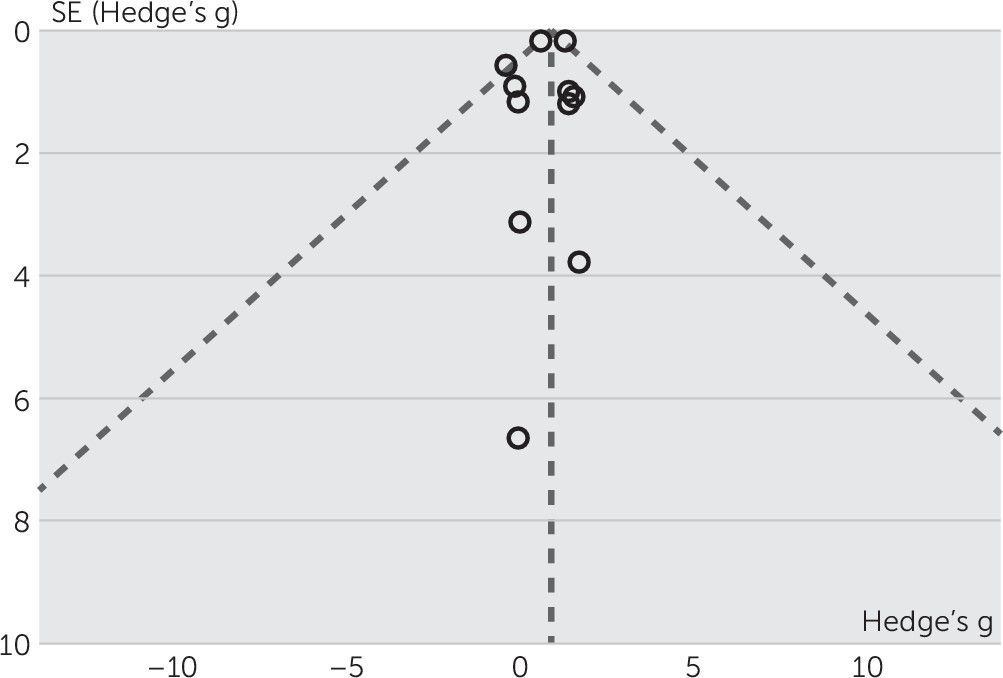
Discussion
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

