Interest in the neural correlates of error monitoring has increased in the past two decades. Two response-locked event‐related potential (ERP) components, error-related negativity (ERN [or Ne])
1–3 and error positivity (Pe),
4 have been identified to be closely related to error monitoring. The ERN is a response-locked ERP component commonly observable 20–100 ms after committing different types of errors, such as commission and omission errors or choice error, irrespective of whether the committed error is consciously perceived.
5 There is an agreement that ERN reflects the activity of a generic response-monitoring system,
6 as well as the unconscious error detection or response conflict, whenever there is a mismatch between the intended and produced responses.
1,5 It has a frontocentral maximum and is thought to be generated by the dorsal part of the anterior cingulate cortex (ACC).
7 The Pe follows the ERN at approximately 200–500 ms after the error has occurred. It has a centroparietal topography and is thought to be generated by the rostral region of the ACC. Several hypotheses on its functional significance can be found in the literature, including a role in conscious error recognition, adjustment of response strategies after committing an error, and the emotional evaluation of the error.
5,8,9Various experimental paradigms, including the choice reaction time task, go/no-go task, or stop task, are used in practice in the research of motor response inhibition impairment in attention deficit hyperactivity disorder (ADHD). Higher commission error rates,
10,11 higher intrasubject variability in reaction time,
12–14 and deficient post-error slowing
15 are the most consistent findings across these paradigms both in childhood ADHD and in adult ADHD. These findings facilitated further research in potential deficits in error monitoring and neural correlates of error processing in ADHD.
The ability to adequately inhibit inappropriate responses in the context of emotional inputs is essential for social functioning. The emotional content of stimuli can interfere with response inhibition and places extra demands on neural resources. Previous studies investigating the influence of long-lasting affective states or traits in healthy individuals found that individuals scoring high on negative affect scales display enhanced ERN amplitudes and decreased Pe amplitudes after commission error.
17,18 Notably, higher impulsivity scores are associated both with lower ERN amplitudes and with lower Pe amplitudes.
19,20 Short-lasting emotional factors have also been shown to modulate ERN amplitude, but the results of previous studies are more inconsistent in this regard. Enhanced ERN amplitude to negative affect induction was reported in two studies
21,22 and in a flanker task for trials with superimposed pleasant pictures compared with unpleasant and neutral ones.
23 In contrast, decreased ERN amplitudes were reported after the presentation of pleasant compared with neutral movie clips prior to a choice reaction time task.
24 In two other studies, the mood and fear induction procedure did not modulate the amplitude of ERN.
25,26In the present study, our aim was to investigate the affective modulation of error monitoring in adult ADHD patients compared with healthy controls. There is growing evidence indicating that besides the cognitive impairments, patients with ADHD frequently manifest deficits in emotion regulation.
27 Although emotional dysregulation is correlated with all the core domains of ADHD, it shares a strong relationship with symptoms of hyperactivity and/or impulsivity.
28–30 Even though researchers have turned to emotional stimulus processing with increasing interest, published data on event-related potentials in emotional processing are scarce, both in adult and in child ADHD literature, and are restricted to stimulus-related ERPs. In our study, we applied a go/no-go task to investigate motor response inhibition in terms of behavioral performance and error-related potentials. Using emotional pictures as stimuli, our goal was to investigate how the affective valence of the stimuli modulates response inhibition and error-related ERPs.
Discussion
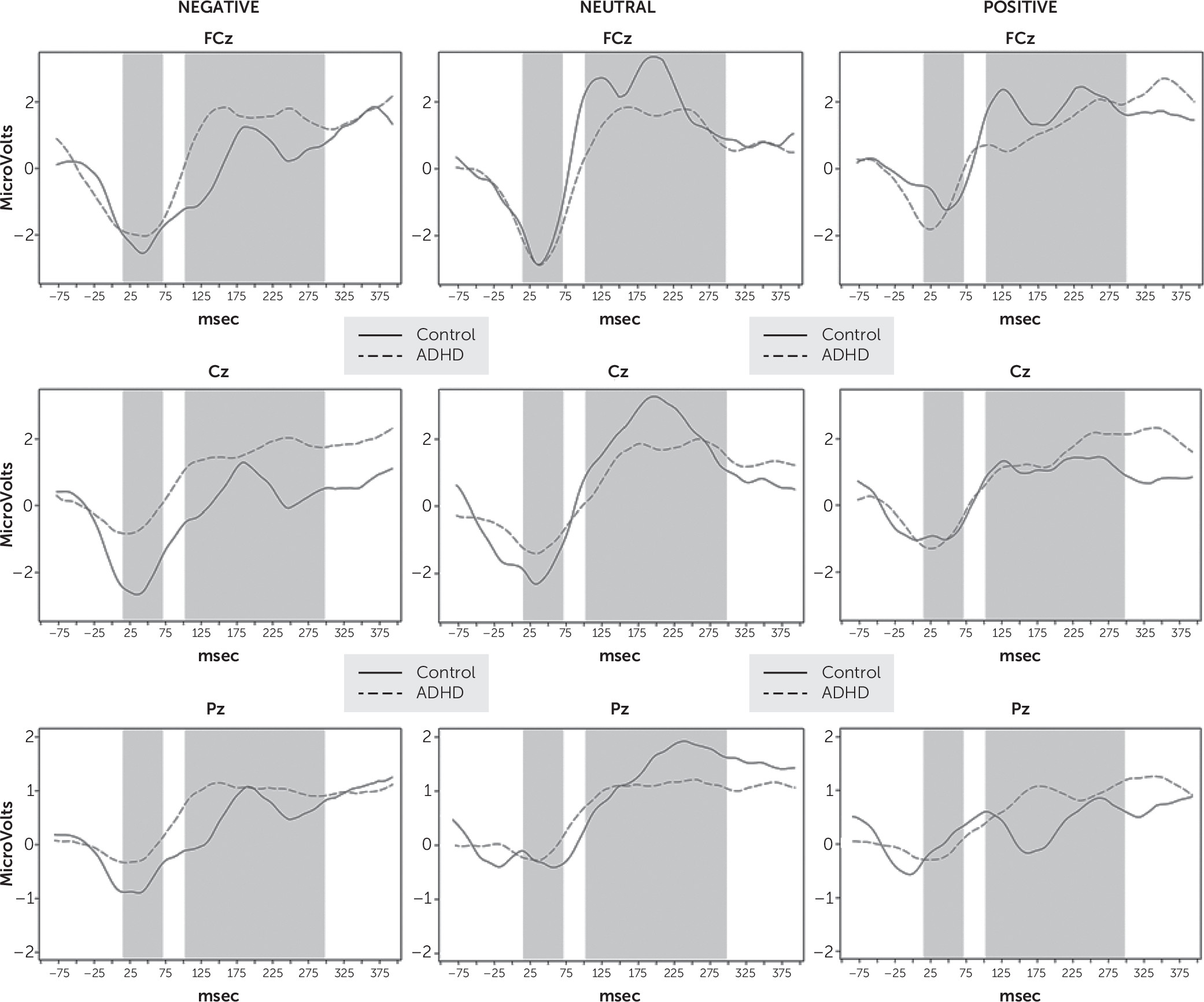
The present study investigated error monitoring after failed inhibition in adult ADHD patients in comparison to healthy controls. To gain a better understanding of how an affective cue modifies the processing of an inhibition error, we applied a go/no-go task with emotional stimuli. We evaluated performance and ERP correlates of error monitoring (ERN and Pe).
Behavioral results showed no difference between the ADHD and control groups in terms of mean reaction time. Comparable data on commission errors in the ADHD literature are limited, as in most studies the applied stimuli had no emotional content. Consistent with the data from the literature available for neutral stimuli,
10,11 in our study the patient group committed significantly more errors for neutral IAPS pictures than the controls. This may be attributable to inhibition impairment; however, since the task requires efforts with regard to working memory and focused attention, it may also be related, at least in part to difficulties with these functions.
In the case of emotionally valenced stimuli, we found a significantly
increased commission error rate in the ADHD group compared with controls for negative stimuli and no significant difference for positive stimuli. The emotional content of the stimuli has been shown to serve as a distractor that captures attention in a bottom-up fashion, thereby disrupting the focus on goal-relevant information.
36 In a study that used induction of short-term affect before the target stimuli,
22 there was a significant main effect of valence: participants responded slower after unpleasant pictures. Considering that there was no significant valence effect on error rates in that study, this finding suggests that processing pictures with negative valence requires more effort; it requires more time to avert an increase in error rate. Due to the attention inhibition deficit, ADHD patients have poor ability to remain focused in the presence of distracting emotional information. The fact that in our study, the ADHD group exhibited similar reaction times to controls but significantly worse performance in response to negatively valenced pictures suggests an enhanced susceptibility and insufficient inhibition with respect to negatively valenced stimuli. Based on our results, this effect seems to be less pronounced when exposed to positive stimuli, and although ADHD patients tended to commit more errors even under such circumstances, there was no significant difference in performance between the two groups.
On a neurophysiological level, the two groups differed significantly in ERN amplitude for negatively valenced stimuli, with significant amplitude reduction in the patient group. For positive stimuli, there were no significant differences, and for neutral stimuli we found a significant ERN amplitude difference at the Cz electrode.
As in prior ERP studies, no stimuli with emotional valence were applied; comparison can only be made with those of our results that were obtained under the neutral condition. In four published studies,
37–40 the authors did not demonstrate a significant decrease in ERN amplitude, while in three studies
41–43 a significant reduction in ERN amplitude was found in the ADHD group compared with controls. The reduction of the ERN in the ADHD participants was consistent across these studies, and the meta-analysis of their data aggregated these statistical findings into a significant result.
16The question of why negative stimuli elicit significantly reduced ERN in the ADHD group arises. One explanation of these findings could be that negative stimuli serve as more potent distractors: by capturing attention they distract from the goal-relevant information. Earlier publications reported of a reduction in ERN amplitude following an error in healthy control subjects during conditions of dual attention constraints.
44–46 In our study, due to the enhanced susceptibility to distractors characterizing ADHD, negative emotional content is expected to distract the attention from the task. Consequently, the error appears as less salient, which in turn could result in a lower ERN amplitude.
It is important to note that in our study, impulsivity was positively associated with higher ERN amplitude in the fronto-central area for the negative condition. Thus, it is possible that the impulsivity/emotional lability and reactivity
47,48 characterizing ADHD mitigates the impact of the aforementioned effect of distraction from goal-relevant information by emotional stimuli during the process of error detection. Further symptom dimensions of CAARS (inattention, hyperactivity, problems with self-concept) showed the same direction as the ADHD group effect; however, statistical significance was reached only in the case of hyperactivity.
We found that patients had significantly lower Pe amplitude than controls in the neutral condition, whereas they did not differ from controls for emotionally valenced stimuli. While the decrease in the Pe amplitude is a consistent finding in child ADHD studies that applied neutral stimuli,
49,50 in adults this observation is much less consistent.
16 Wiersema et al. reported a significant reduction of Pe in 23 adult ADHD patients in a visual go/no-go task and a negative correlation with the ADHD symptom severity as measured by the Adult Self-Report scale and the Wender Utah Rating Scale.
39 However, the ADHD subjects and matched controls showed no significant differences in terms of behavioral results, such as reaction time, accuracy, and post-error slowing. Herrmann et al. investigated a younger (mean age=25.2 years) and an older subgroup (mean age=40.9 years) of ADHD patients and control subjects.
42 Commission error rate and post-error slowing were significantly higher only in the younger ADHD subgroup compared with the age-matched controls, while reduced Pe amplitude was found for the whole ADHD sample. With increasing age, improved performance was found among the ADHD patients for both aforementioned behavioral measures, while no effect of age was observed for controls. For the Pe amplitude, no group interaction was present with age. These findings emphasize the importance of developmental factors in ADHD and suggest that as opposed to the impairments in behavioral measures, the amplitude reduction of Pe is a stable impairment that persists over time.
51In our study, patients did not differ from controls in terms of Pe amplitude for emotionally valenced stimuli. Available data indicate that the magnitude of the Pe amplitude is proportional to the extent of awareness. When accuracy is emphasized in the instructions given for solving a task, the Pe amplitude increases.
52 One explanation for this could be that even though the emotional valence of the stimulus served as distracting information, it reduced the monotony of the task. Furthermore, by positively affecting the motivational components in ADHD patients, it resulted in increased awareness of the errors committed and improved attempts at following instructions. Higher monotony of neutral pictures results in a more pronounced deficit in error monitoring as indicated by the significantly lower Pe observed in the ADHD group.
A limitation of our study is the small sample size in the control group as a result of a lower rate of qualification for the analyses, which was due to difficulty in enrolling control subjects with a sufficient number of error trials for the investigation. Furthermore, IAPS images were not matched on arousal ratings, and negative pictures in general are associated with higher arousal than the neutral or the majority of the positive pictures. However, balancing the pictures across emotion categories for arousal is difficult and may lead to selection bias (e.g., use of pictures with specific semantic categories). The ADHD sample included a subgroup of patients who were medicated with psychostimulants. This limits generalizability with regard to unmedicated samples despite the fact that patients discontinued their medication 24 hours prior to the ERP study. Notwithstanding these limitations, our study is the first, to our knowledge, to investigate the neurobiological basis of how affective cues modify cognitive control, response inhibition, and the error monitoring in adult ADHD patients.
In conclusion, behavioral performance and ERP correlates of error monitoring (ERN and Pe) are a prominent field of adult ADHD research. There is growing evidence indicating that besides cognitive impairments, patients with ADHD have deficits in emotional processing and emotion regulation, although the number of studies investigating the association between these areas is rather limited. In our study, the Pe amplitude decreased significantly in the ADHD group when we applied neutral stimuli, and the ERN amplitude showed a reduction for stimuli with negative emotional valence. While these results are in line with previous results in the literature, they underline the need to further investigate how the emotional content of the stimuli interferes with the process of error monitoring in ADHD. Beyond behavioral data, electrophysiological examination of error monitoring is essential for the characterization of the neurobiological basis of the self-monitoring deficit characteristic in ADHD.