Alcohol use disorders are among the most prevalent and commonly comorbid mental health conditions. Chronic alcohol use is associated with many behavioral concerns, including complaints with sleep in 35%−91% of patients.
16,17 The most common problems include increased sleep latency (i.e., difficulty initiating sleep), poor sleep quality, and daytime sleepiness. Often, these sleep problems will persist through withdrawal but are expected to resolve following prolonged abstinence.
18 The effects of alcohol on sleep have detrimental socioeconomic consequences, as alcohol associated sleep problems account for approximately 10% of the annual costs related to alcohol use disorders.
19,20 The DSM-5 defines alcohol use disorder as a problematic pattern of alcohol use that leads to the presence of at least two pervasive social (e.g., neglecting social functions), functional (e.g., use when physically hazardous), or health (e.g., tolerance and/or withdrawal) impairments/symptoms that occur within a one year time span.
21 This can differ from long-term heavy alcohol use that is present, but not problematic. Research studies vary considerably in their criteria for alcohol use and abuse, so the collective term “chronic alcohol use” will be utilized.
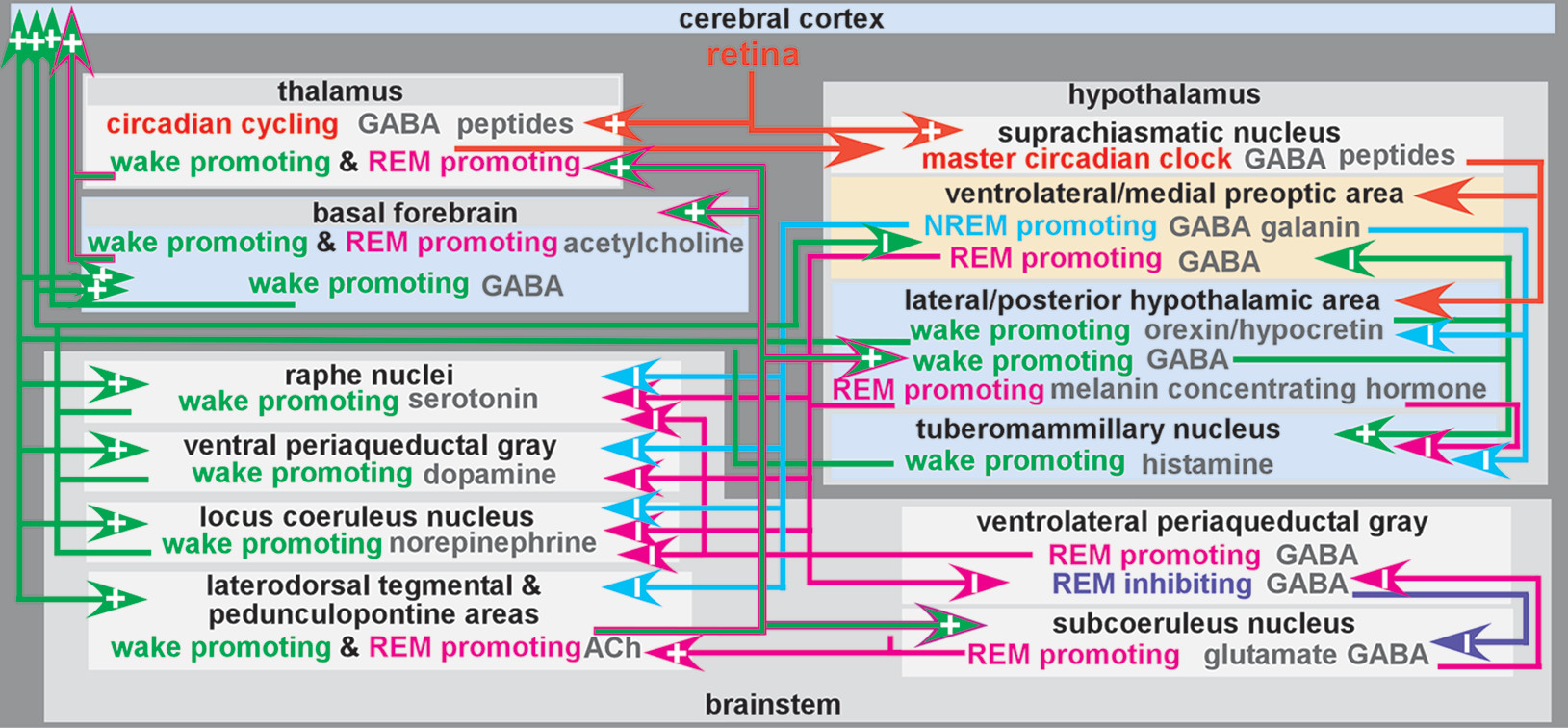
The normal mechanisms of sleep-wake cycling are largely regulated by a balance of homeostatic drive promoting sleep integrated with the circadian system that maintains timing of wakefulness and sleep in the day-night cycle (
Figure 1).
1,2,4,5,22–24 The sleep-wake circadian rhythm is a biological process sustaining an endogenous oscillation of approximately 24 hours when driven by light and darkness in the environment. Sleep and circadian rhythms are bidirectionally linked and have substantial overlap between and integration of underlying neurobiological systems.
22,25 The suprachiasmatic nucleus in the anterior hypothalamus is the master circadian clock in mammals.
26 Circadian rhythms generated by the suprachiasmatic nucleus are entrained to the daily light-dark cycle. Daily phase resetting is mediated by input from specialized (melanopsin containing) retinal ganglion cells. There is also extensive overlap between the circadian clock and stress response systems, such that the hypothalamic-pituitary-adrenal axis and glucocorticoid systems are temporally controlled by the circadian clock.
27Sleep drive gradually intensifies during wake time and dissipates during sleep. One contributor to sleep drive is buildup of sleep modulating substances (i.e., sleep factors) during the waking period in multiple brain regions.
2,28 Several sleep factors have been identified (e.g., adenosine, nitric oxide, tumor necrosis factor alpha, interleukin 1).
2,5,20 Adenosine has been of particular interest because its formation is related to neuronal activity and caffeine is an adenosine A
1 receptor antagonist.
2,5,20 It also may serve as a final common pathway for other sleep factors.
5,20 Adenosine accumulation promotes sleep by inhibiting (via A
1 receptors) wake-promoting neurons in the basal forebrain and lateral hypothalamus and by exciting (via A
2 receptors) sleep-promoting neurons in the ventrolateral preoptic area of the hypothalamus (
Figure 1).
5,20,29,30 This wakefulness-dependent increase in adenosine peaks before sleep onset. It is thought to promote sleep onset by inhibiting ascending arousal-related projections of the brainstem and basal forebrain regions (
Figure 1). During daylight hours, circadian arousal-promoting mechanisms counter the sleep drive to maintain wakefulness. This influence subsides as evening approaches and sleep drive peaks. Nonrapid eye movement (NREM) sleep early in the sleep period will be greater with higher adenosine accumulation. Adenosine will dissipate throughout the sleep cycle as rapid eye movement (REM) sleep commences.
31,32 Within the homeostatic sleep drive, an ultradian rhythm regulates the approximate 90-minute cyclical alteration of NREM and REM sleep.
33Preclinical studies have provided some insights into ways that alcohol may interact with components of the sleep-wake system. Adenosine is implicated in the pathophysiology of both sleep disorders and chronic alcohol use.
34 Alcohol administration induces an increase in extracellular adenosine level in some brain regions, resulting in increased inhibition.
35 Studies in cultured cells indicate that acute alcohol exposure inhibits transporter mediated (equilibrative nucleoside transporter 1, ENT1) reuptake of adenosine. Research with rodents suggests that alcohol induces dose-dependent adenosine accumulation in the basal forebrain that inhibits wake-promoting neurons.
36 Downregulation of both ENT1 and A
1 receptor expression in the basal forebrain has been demonstrated during acute withdrawal following development of alcohol dependency.
35,37,38 As noted by these authors, insomnia during withdrawal may be due in part to reduced inhibition of basal forebrain wake-promoting neurons disrupting sleep homeostasis. Acute administration of alcohol enhances inhibition by both increasing GABA activity (e.g., activating receptors, facilitating release) and decreasing glutamate activity (e.g., inactivating receptors), likely mediating some of the sedative properties.
17,19 It has been suggested that a primary causal mechanism of increased NREM sleep following acute alcohol consumption is the inhibition of wake-promoting neurons through activation of GABA
A receptors. Decreased REM sleep may be due to the activation of GABA
B receptors and/or inhibition of kainate receptors on brainstem cholinergic cells.
19 Chronic exposure to high doses of alcohol induces adaptive changes (e.g., decreased inhibitory response of GABA
A receptor systems, increased activity of excitatory glutamate receptor systems), which may contribute to tolerance to the sedative effects.
17,19 In addition, six weeks of chronic exposure to alcohol is sufficient to induce desynchrony among circadian rhythms (e.g., core body temperature, activity level, hormonal secretion) and phase shift of peripheral circadian clocks.
39In laboratory studies, ingestion of a moderate amount of alcohol before bedtime in healthy individuals is typically associated with decreased sleep latency, increased NREM sleep, increased total sleep time, and reduced or fragmented REM sleep that leads to decreased sleep efficiency.
17,19,20,40,41 This is followed by rebound increases of REM sleep on subsequent nights.
42,43 Similarly, a longitudinal community study (months) that utilized multilevel modeling to examine associations between daily alcohol use and each night’s sleep found a positive association with sleep duration and a negative association with sleep quality.
44 Of note, the range for within-person association was wide, indicating considerable individual differences to the effects of alcohol use. It has been suggested that tolerance to the sleep-enhancing effects of alcohol can develop quickly (e.g., 3 to 9 nights of daily use), although this likely varies by person.
17,45 Alcohol use before bedtime can also disrupt circadian rhythms.
25,46 Decreased melatonin secretion and altered core body temperature rhythm are the most frequently reported effects.
25Sustained chronic abuse of alcohol may result in detrimental changes in multiple aspects of sleep. Chronic alcohol use is associated with longer sleep latency, altered NREM sleep, decreased and disrupted REM sleep, and reduced total sleep time.
19,41,47 These changes occur as individuals who use alcohol chronically become tolerant to the sleep-enhancing effects of alcohol, but remain sensitive to the stimulating effects.
19,48 During periods of heavier drinking (e.g., binge drinking), chronic users may experience reduced sleep latency and increased NREM sleep, similar to acute effects of alcohol in healthy individuals.
48 Sleep duration is still typically shortened overall. Although there is limited research on changes to circadian rhythms associated with chronic alcohol use, the most commonly reported finding is altered melatonin secretion (e.g., elevated daytime levels, delayed phase).
25,46,49 Based on the known interactions among these systems, it is likely that the hypothalamic-pituitary-adrenal axis is involved in mediating some of the negative effects of chronic alcohol use on sleep and circadian rhythms.
39Most human studies evaluating the effects of chronic use of alcohol on sleep focus on acute and postacute withdrawal. During acute withdrawal, sleep latency increases and total sleep decreases (relative to drinking periods), congruent with the development of tolerance to the hypnotic effects of alcohol.
16,19 Acute withdrawal is also associated with a decrease in NREM sleep, more frequent REM episodes, reduction of REM latency, and shortened NREM-REM cycles, but without increased total REM duration.
47,50 Higher REM sleep deficit is typically seen in abstinent individuals compared with healthy controls, and it has been suggested that this may be both a pre-existing risk factor for chronic alcohol use as well as for relapse.
17 Although NREM sleep will improve over time abstinent, reports are varied as to how and when specific stages of NREM sleep stabilize.
16,45,51 Early research suggested that stage one NREM sleep stabilizes after a year, stage three within several months, and reports of stage four sleep are variable (use of the term stage four sleep has been discontinued due to the unclear distinction between sleep stages three and four).
16,52 Altered melatonin phase timing appears to normalize with abstinence, although it may be worsened temporarily during acute withdrawal.
25,49,53,54 Persistent sleep problems are marked by reduced amounts of NREM sleep and shortened sleep times overall with prolonged disorganization of circadian rhythms, the homeostatic sleep system, and disruptions of transitions between sleep stages.
16,25Autopsy studies have reported that uncomplicated chronic alcohol use is associated with mild brain atrophy, primarily due to decreased white matter, with clear evidence of cellular loss only in lateral prefrontal cortex.
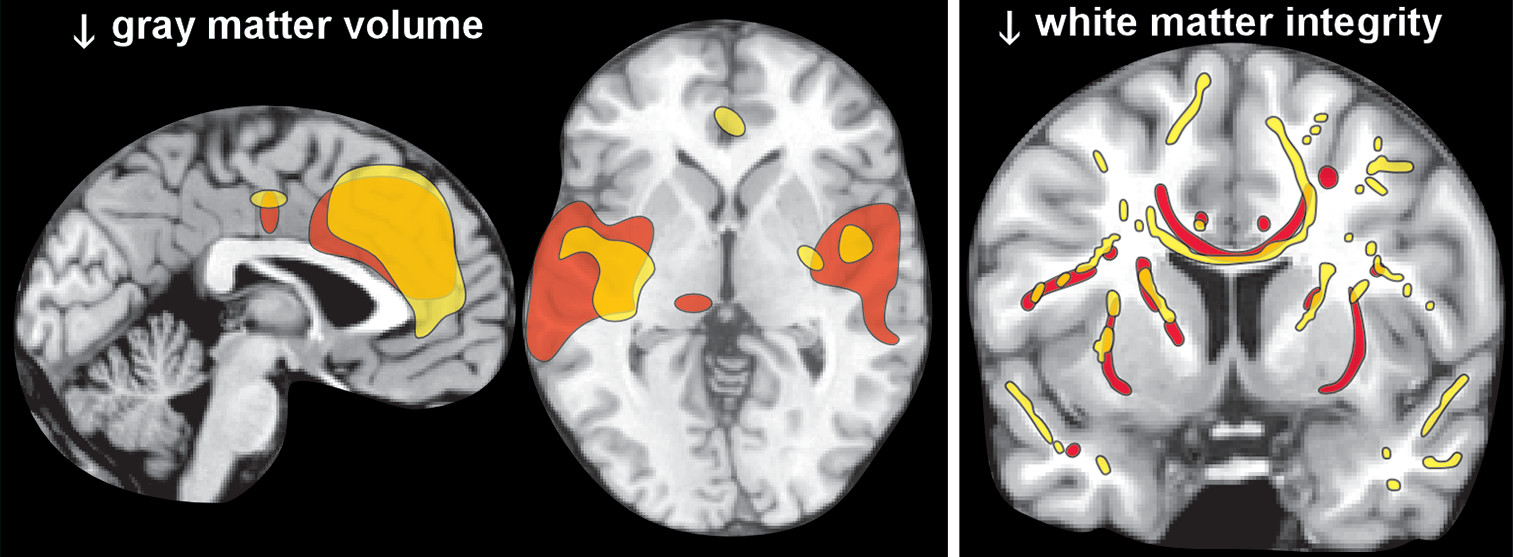
6,7 In contrast, magnetic resonance imaging (MRI) studies have reported both decreases in white matter and widespread areas of cortical thinning and/or reduced gray matter volume.
6,7 Two meta-analyses of voxel-based morphometry studies that compared a group with uncomplicated chronic alcohol use to healthy controls found mostly similar areas with reduced gray matter volume (
Figure 2).
8,9 A weakness acknowledged by one was that they were unable to incorporate duration of abstinence, which varied considerably across studies, into their analytics.
8 A meta-analysis of MRI studies that compared groups with chronic alcohol use to healthy controls found that both treatment seeking status and number of days abstinent were significant moderators of white matter volume.
55 Non-treatment-seeking groups did not differ significantly from healthy controls and the differences in volume between groups with chronic alcohol use and healthy controls decreased as days abstinent increased. More recent studies in individuals with chronic alcohol use entering treatment have also reported that gray matter metrics (total and regional volumes; cortical thickness) were initially smaller (first week of abstinence; first day of abstinence) compared with healthy controls, but white matter volumes were not.
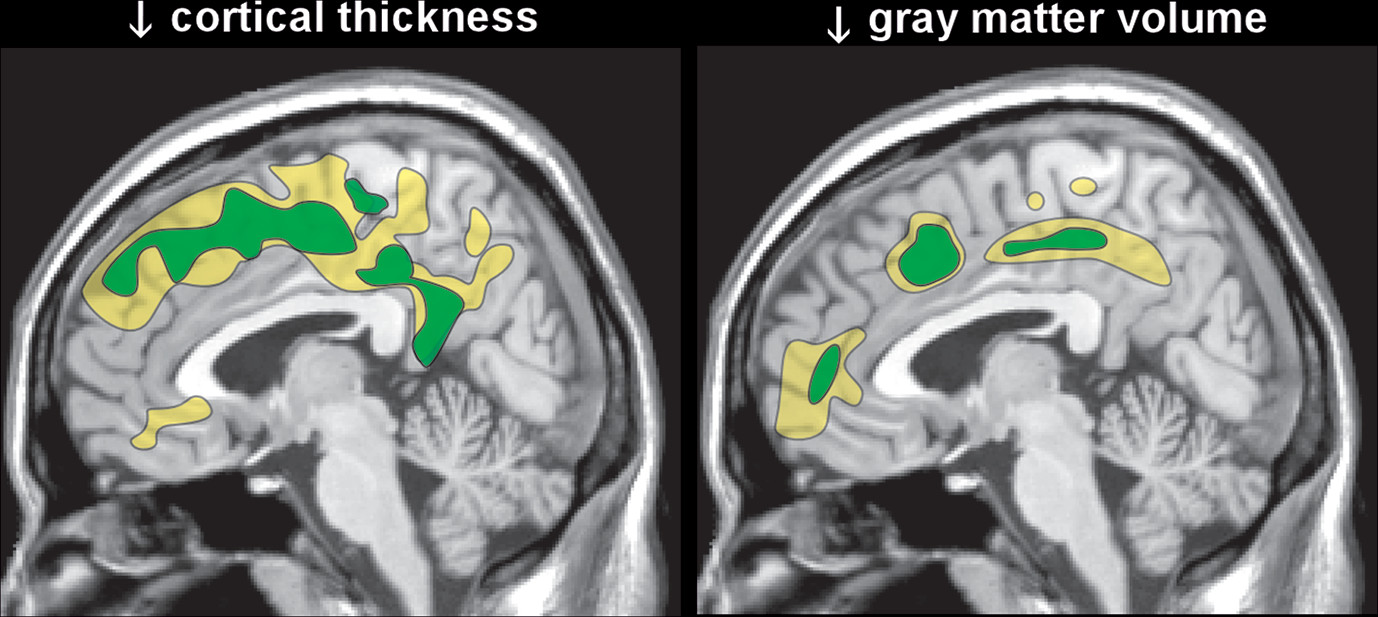
12,13 One group reported significant recovery during the first two weeks of abstinence in both gray matter volume and cortical thickness (
Figure 3).
13,15 The other reported that volumetric increases with abstinence (1 and 7.5 months) were seen in both tissue compartments, with gray matter increasing earlier than white matter.
12 Smoking was associated with less recovery in total and frontal gray matter volumes.
12 Diffusion tensor imaging has been used to assess white matter integrity in groups with chronic alcohol use compared with healthy controls. Studies performed in early abstinence have found widespread areas of impaired integrity (lower fractional anisotropy) compared with controls (
Figure 2).
10,11,14 Longitudinal studies indicate that prolonged abstinence is associated with partial normalization of white matter integrity.
11,14 Studies suggest that recovery may also occur without total abstinence, which supports reduction efforts for treating alcohol problems when complete abstinence may not be realistic.
10,56The evidence that alterations in the brain associated with chronic alcohol use can improve measurably in as little as two weeks of abstinence indicates that the brain has a robust ability to repair and/or restructure.
6,7 Multiple mechanisms have been proposed for reduced tissue volumes in both gray and white matter including inflammation, oxidative stress, myelin thinning, cell body shrinkage, disruption of intracellular cytoskeletal structure/functioning, dendritic pruning, and suppression of neurogenesis.
7,51,57–59 Alcohol-induced liver dysfunction and repeated episodes of subclinical nutritional deficiencies (e.g., thiamine) are also likely to be relevant. Once thought to be important for the reversibility of atrophy due to chronic alcohol use, the rehydration hypothesis has been disproven.
6 Although still in the early stages of investigation, processes that may contribute to volumetric recovery include remyelination, regrowth of dendrites, and compensatory increases in neurogenesis.
13,58 Longitudinal studies have reported residual differences between chronic alcohol users and healthy controls. Although some of these differences may be indicative of permanent alcohol-related changes, it is likely that greater recovery will be found with increased time abstinent. In addition, there is growing evidence of pre-existing differences that are risk factors for the development of chronic alcohol use.
60,61Problems with sleep have been implicated as a risk factor for both development of problematic alcohol use and relapse during abstinence.
17,41,62 Cross-sectional studies in adolescents have reported that sleep-associated factors (e.g., poor sleep habits, evening chronotype, circadian phase delay) correlate with development of many problems including alcohol misuse.
17 More recent longitudinal studies of adolescents also support problematic sleep as a risk factor.
63–65 In addition, decreases in sleep quality longitudinally predict problematic alcohol use in Navy Veterans following deployment.
66 Increase in REM sleep deficit during abstinence has been noted as a possible predictor of vulnerability to relapse.
67 Abstinent individuals who have difficulties falling asleep may also be at higher risk for relapse as acute alcohol consumption reverses NREM deficits and decreases sleep latency.
16,68 This interpretation is consistent with a recent follow-up study of patients who completed an intensive residential treatment program.
69 Both use of hypnotic medications and use of alcohol as a hypnotic at time of admission were associated with relapse by 12 months, but sleep problems at admission or discharge were not. Prioritizing treatment of sleep problems in patients with primary or comorbid chronic alcohol use may reduce the risk of relapse. Early preventative intervention is also pertinent, as sleep problems in adolescents predict later alcohol misuse.
17Both pharmacologic and behavioral options are available to treat sleep problems (e.g., insomnia).
41,62,70,71 Evidence regarding the efficacy of using medications to treat sleep problems in this population varies considerably.
41,70,71 Trazadone is one of the most commonly prescribed medications for sleep problems in chronic alcohol use.
70,71 A recent review of nonpharmacologic approaches to treating sleep problems in patients with alcohol related disorders identified only five relevant studies.
62 The study utilizing progressive relaxation training reported improvements in sleep quality. Four studies utilizing cognitive behavioral therapy for insomnia (CBT-I) reported improvements in multiple aspects of sleep and decreases in daytime fatigue. In some instances, CBT-I has been noted as beneficial in helping wean patients off of medication for insomnia.
72 However, the efficacy of CBT-I for reducing relapse is unclear.
73In summary, most evidence suggests that chronic alcohol use affects homeostatic sleep drive most prominently, as indicated by significant alterations in NREM and REM sleep, as well as prolonged sleep latency. Although sleep systems are expected to improve with abstinence, previous chronic alcohol use may be a risk factor for relapse in patients presenting with sleep problems. Recent longitudinal studies indicate considerable recovery in gray and white matter volumes with abstinence.