Prolactinomas represent one-third of all pituitary adenomas and are the most common secreting pituitary tumor.
1 Signs and symptoms include altered menses, galactorrhea, and mass effect on surrounding structures such as the optic chiasm. Treatment goals are restoring sexual and gonadal function, preserving pituitary function, reduction of the tumor, and preventing progression and recurrence by normalizing prolactin levels.
1 Treatment modalities include dopamine agonists (DA), neurosurgery, and radiotherapy. DA are the gold-standard treatment regardless of tumor size.
2 Side effects are less common with cabergoline but include gastrointestinal, neurological, and cardiovascular symptoms.
1 De novo psychosis arising from the use of DA is rare, has mostly been reported as presenting or exacerbating in patients with a familial or personal history of mental illness, and its treatment remains a clinical challenge.
3–5We present the case of a woman with no familial or personal history of mental illness who was treated successfully with clozapine for psychosis secondary to the use of cabergoline in the treatment of a giant prolactinoma.
Case Report
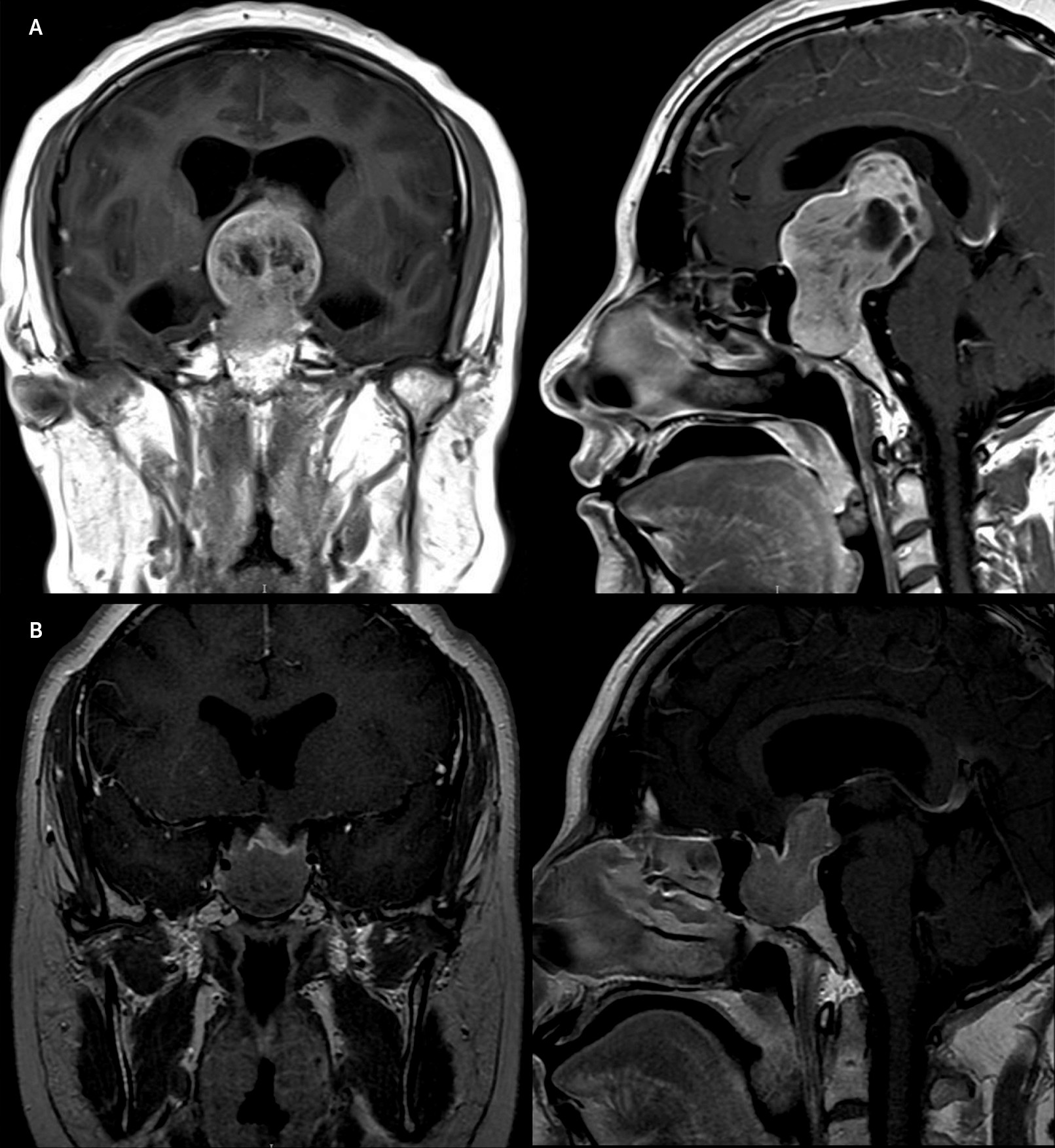
A 31-year-old woman presented with a 4-year history of amenorrhea, progressive visual field disturbances, and recent onset of headache. She had no relevant past medical or psychiatric history. As part of the work-up, contrast-enhanced MRI was obtained, which revealed a 46×27×37-mm pituitary cystic adenoma with suprasellar extension involving the optic chiasma, ventromedial cortex, subgenual cingulate cortex, and lateral ventricles (
Figure 1A). Visual field testing showed compressive optic neuropathy, sparing only the lower nasal quadrants of vision. A diagnosis of giant prolactinoma was made, with a diluted prolactin level of 13,494 ng/mL, hypogonadotropic hypogonadism (follicle-stimulating hormone: 4.32 mIU/mL, luteinizing hormone: 1.08 mIU/mL, and estradiol: 10 pg/mL). The patient’s thyroid function tests were normal (IGF-1 was 79.7 ng/mL), and central adrenal insufficiency was ruled out.
Cabergoline dose was initiated at 0.25 mg weekly for 2 consecutive weeks. Two days after starting treatment, the patient began to exhibit behavioral changes associated with paranoid delusions and auditory hallucinations involving a drug cartel chasing her. The symptoms faded over the course of the week to begin again after the next dose, and they were more intense when reaching 0.5 mg per week. When reassessed after 2 weeks of treatment, her prolactin levels declined to 5934.9 ng/mL, and visual improvement was evidenced.
The patient was admitted to the neuropsychiatry department for behavioral management and treatment adjustment. Upon neuropsychiatric examination, she displayed evasive behavior and expressed paranoid and referral delusions involving the medical team, as well as thought control delusions regarding her father. She believed that the medical team would set her up in order to obtain information regarding her sexual orientation. She also believed that her father had the ability to control her family’s will in order to limit her medication, and she stated that people thought that she had harassed her nephew. Psychosis caused acute distress and agitation that required admission. After interdisciplinary deliberation with the neuroendocrinology department, clozapine was started at 12.5 mg and titrated up to 50 mg daily. The patient’s psychosis responded after receiving 25 mg, and when a 50-mg dose was reached, her neuropsychiatric symptoms disappeared. Cabergoline was not suspended.
After a 2-month follow-up, prolactin levels diminished drastically (prolactin: 95.7 ng/mL), the volume of the prolactinoma decreased significantly (
Figure 1B), and visual field deficits improved. The patient’s psychosis, while on cabergoline and clozapine, was still in remission, and she had returned to her previous functionality. A complete blood count, as well as other tests were performed weekly to ensure no signs of side effects due to clozapine were present.
Discussion
Psychosis is a rare side effect of DA. Cabergoline’s potential to cause or exacerbate psychosis has been reported in few cases when treating prolactinomas and is more frequent in patients with previous personal or familial history of mental illness.
3–5Psychosis has been associated with dysregulation of fronto-striato-thalamic circuits.
6 It remains a question whether DA only can account for the neuropsychiatric profile of our patient or whether a mass effect on these loops serves as a predisposing factor for the presentation of this side effect.
Treatment options for prolactinomas include surgery, radiotherapy, and medical treatment. Indications for surgery include intolerance or resistance to medical treatment and patients requiring chronic antipsychotic medications.
1 Other DA such as quinagolide, pergolide, or somatostatin receptor 5 agonists like pasireotide may be an option;
2 however, they may not be available or affordable in some cases.
Treatment for patients presenting with psychosis and prolactinomas should consider multiple individual features. Based on the extension of the prolactinoma at presentation and its response to medical treatment after reassessment, cabergoline was continued while adding clozapine for its low effect on prolactin, growth of prolactinomas, and potent antipsychotic properties.
7,8 Other approaches to this particular presentation have been suggested with aripiprazole and quetiapine.
7–9Clozapine may be effective and safe in patients with carbergoline-induced psychosis, without need for DA suspension or surgical removal. Few previous reports have explored its utility when other DA such as bromocriptine are used for treating prolactinomas.
7,10 We considered that it was the best option for our patient because of its limited cost and wide accessibility in our institution. This case suggests that psychosis may occur independently of cabergoline dose and duration of treatment and the presence of previous mental illness. Additionally, psychosis can be treated successfully with clozapine, without discontinuing DA.