According to national estimates, the proportion of Americans reporting current cannabis use,
1 and the proportion seeking treatment for primary cannabis use,
2 are both increasing. Despite these trends, there are no U.S. Food and Drug Administration (FDA)-approved medications for treating cannabis use disorders (CBUD). Medication development efforts have primarily focused on attenuation of cannabis withdrawal symptoms. Many theories of addictions, however, also suggest that by disrupting executive control functions of the prefrontal cortex (PFC), repeated drug use can produce behavioral changes that ultimately underlie the difficulty that many individuals have in resisting habitual drug use once established.
3 Consistent with this, among cannabis users, impairments of PFC functions have been shown to predict poor treatment outcomes in pharmacotherapy
4 and psychotherapy
5,6 studies. In fact, numerous epidemiological studies support the idea that cannabis use onset at an early age increases risk for the later development of cannabis use problems.
7–10An age-dependent, dose-response relationship exists between prior cannabis use and impaired executive functions.
11 For example, longitudinal
12–14 and cross-sectional studies
15 suggest that in comparison with adult-onset users, adolescent-onset cannabis users exhibit more severe and more persistent cognitive impairments. Neuroanatomical and neurophysiological studies in adolescent-onset cannabis users,
16–19 and well-controlled studies in adolescent monkeys,
20,21 provide additional evidence that the still-maturing PFC
22–24 is particularly vulnerable to the adverse effects produced by cannabis. Furthermore, that the majority (81%) of admissions to substance abuse treatment services for primary cannabis use initiated by age 16
2 also supports the hypothesis that cannabis use during the protracted developmental period of adolescence increases risk for the later development of cannabis use problems by disrupting normal maturation of the PFC. These studies collectively support the idea that normalizing PFC-mediated functions affected by cannabis is a reasonable pharmacological strategy for improving CBUD treatment outcomes.
While cannabinoid-1 receptors colocalize with,
25 and cause functional desensitization of, α-2A adrenoceptors
26,27 in PFC, stimulation of postsynaptic α-2A adrenoceptors normalizes PFC functions.
28 Guanfacine, which preferentially stimulates α-2A receptors in PFC,
29 has been shown to improve executive functions in healthy volunteers,
30 individuals with schizophrenia,
31 and children with attention-deficit hyperactivity disorder.
32 Medications targeting α-2A receptors have been proposed as a promising pharmacotherapy target for addiction, not only because of beneficial effects on cognitive functioning related to drug use behavior but also because of the ability of this medication class to decrease noradrenergic hyperactivity and symptomatology associated with cannabis withdrawal and relapse.
33–39 We therefore conducted a double-blind, placebo-controlled crossover pilot study to examine the effects of short-term treatment with guanfacine on cognitive task performance following acute administrations of ∆-9-tetrahydrocannabiol (THC) and on laboratory-induced withdrawal in adolescent-onset, cannabis-dependent individuals (withdrawal data will be published separately from the data presented in this study). We focused here on evaluating the influence of guanfacine on cognitive processes that are adversely affected by acute THC administration and hypothesized that guanfacine would attenuate THC-induced adverse effects on cognitive functions mediated by the PFC.
Methods
This double-blind, placebo-controlled crossover study was approved by the Baylor College of Medicine and the Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) institutional review boards. Additionally, this material is the result of work supported with resources from, and the use of facilities at, the MEDVAMC (Houston, Tex.). All volunteers provided written informed consent after being apprised of the potential risks of study participation.
Participants
Recruited through advertisements, participants were nontreatment-seeking, English-speaking volunteers between 18- and 55-years-old. Study volunteers self-reported a history of using cannabis prior to age 18, provided urine samples that were positive for carboxy-THC (but negative for other illicit drugs), and met criteria for CBUD. Potential participants were excluded if they had a history of seizure disorder, head trauma, drug dependence (except nicotine), adverse reactions to study medications, psychoactive medication use, or other axis I psychiatric disorders. Serious medical conditions, including heart disease, symptomatic HIV-related disease, and asthma were exclusionary. Women were excluded if they were pregnant, breastfeeding, or not using a reliable form of birth control. Participants were paid for their participation.
General Procedures
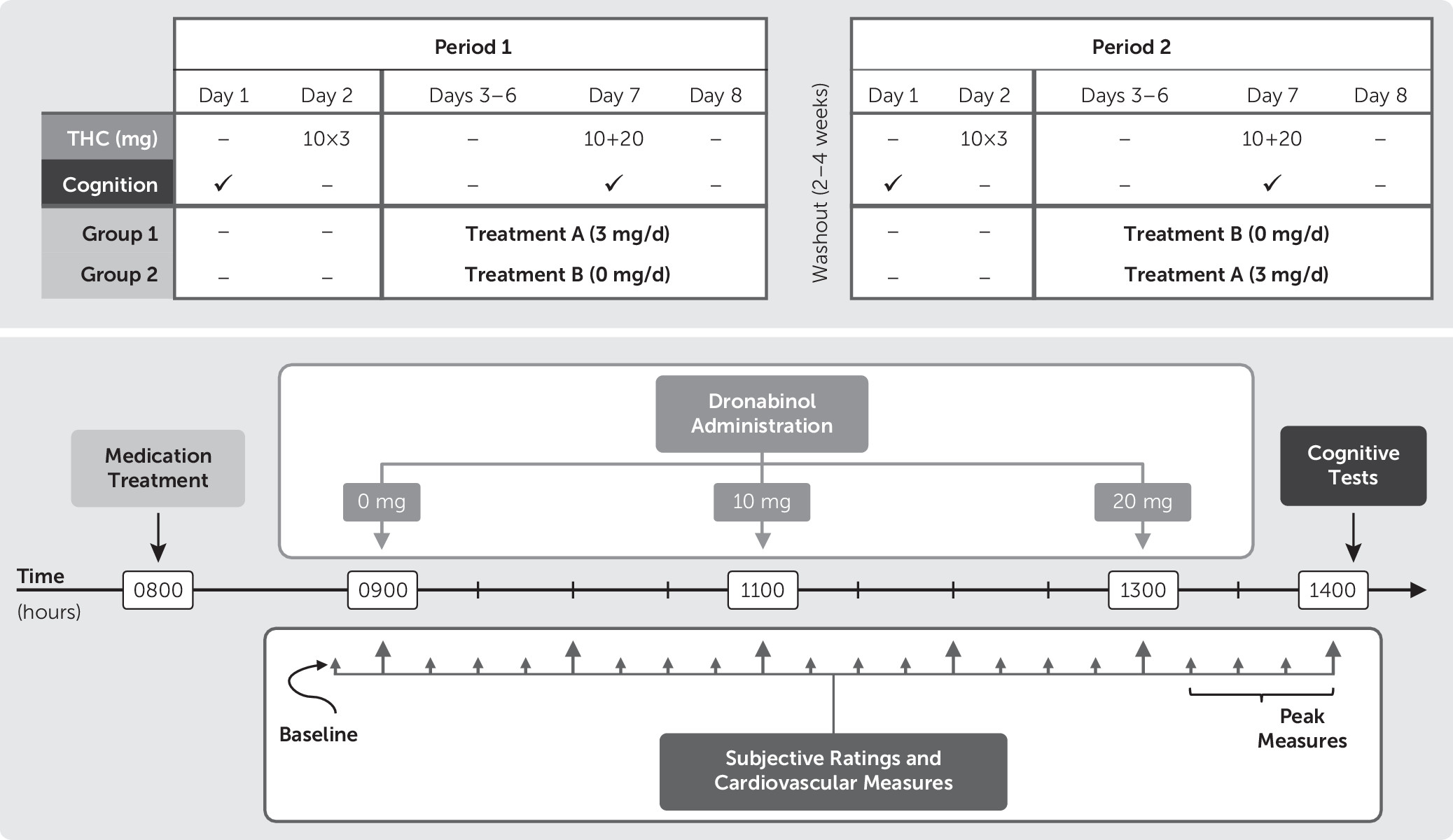
The FDA approved an investigational new drug application for the use of dronabinol and guanfacine in this study. Participants were housed on the Research Commons at the MEDVAMC during each of the two 8-day study periods. Participants completed identical procedures during both study periods, which were separated by 2–4 weeks. On the day of admission (day 1) at approximately 1400 hours, participants completed the cognitive battery. On day 2, participants received 10-mg THC capsules at 0900, 1100, and 1300 hours, to standardize final THC exposure before the onset of abstinence
39 and allow for comparative assessment of withdrawal data during abstinence. On days 3–8, participants received either guanfacine or matching placebo (masked by encapsulation) with the order counterbalanced across participants. Guanfacine was titrated up by 1 mg per day; hence, participants received 1 mg on day 3, 2 mg on day 4, and 3 mg on days 5–8. On day 7, THC (0, 10, and 20 mg) capsules were administered, single-blinded, in ascending order at 0900, 1100, and 1300 hours; participants completed the cognitive battery, 1 hour after the 20-mg dose.
Figure 1 provides an overview of the crossover design and timeline of day 7 events.
Cognitive Tests
Participants completed the revised Hopkins Verbal Learning Test (HVLT), the dual n-back task, and the Continuous Performance Test-II (CPT), as previously described.
40,41 Standardized instructions were provided, both oral and written, prior to administration, and participants were instructed to respond as quickly and as accurately as possible.
HVLT.
Immediate recall, short-term learning, and delayed free recall were measured with the HVLT.
42 For this study, after the three consecutive, immediate free-recall trials of a 12-item, semantically categorized list, the dual n-back test was completed. Following the dual n-back task (approximately 25 minutes), the delayed free-recall trials of the HVLT were completed. To minimize practice effects, we administered six different but parallel versions of the HVLT.
Dual n-back.
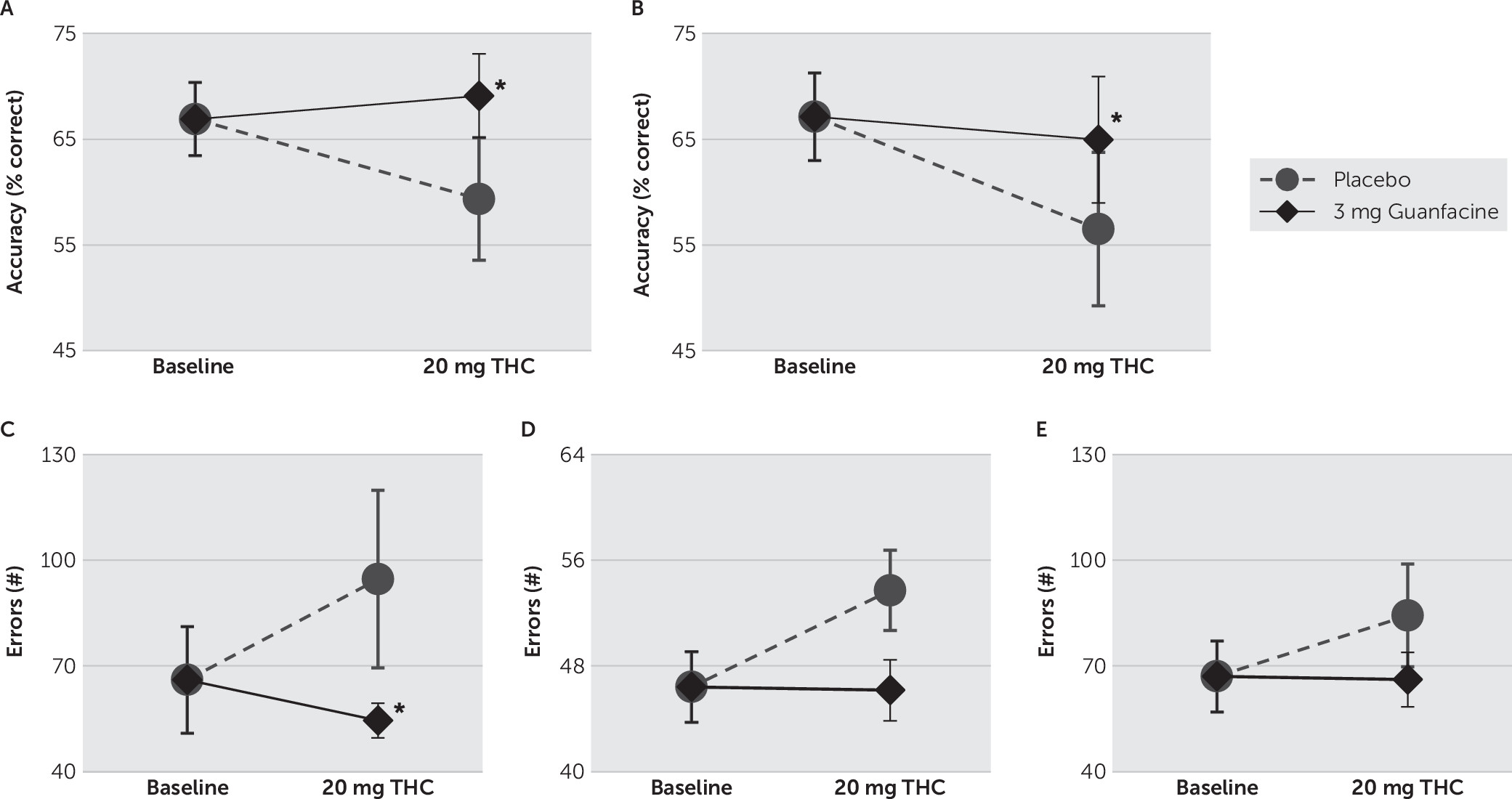
Working memory was measured using the dual n-back task.
43 In this task, spatial locations were cued successively at eight different locations on a computer monitor at a frequency of one cue per 3 seconds (stimulus duration of 500 ms; interstimulus interval of 2500 ms). Simultaneously, auditory cues (one of eight consonants) were presented through headphones, in sequence with the presentation of spatial locations. There were 20 blocks, of 20 trials each, presented over approximately 25 minutes. Responses, made on a standard keyboard, were required whenever one or both stimuli matched a stimulus presented n-positions back in the sequence. No responses were required for nonmatching stimuli. The level of difficulty (i.e., n-value) was always the same for both stimulus modalities. The n-value varied per an individual’s performance after each block of 20 trials.
44 That is, performance was determined after each 20-trial block by the program, and the n-value was adjusted per the following criteria: (1)if a participant responded correctly on ≥85% of the trials, the n-value increased by 1; (2)if a participant responded correctly on ≤70% of the trials, the n-value decreased by 1; otherwise, (3) the n-value remained unchanged.
CPT.
Following the delayed free-recall trials of the HVLT, we measured vigilance, distractibility, and impulsivity using the CPT.
45 In this task, participants attended to letters presented (250 ms) sequentially on a monitor at intervals of 1, 2, and 4 seconds. Participants were instructed to press the space bar on a standard keyboard as quickly as possible after each letter, except when the letter “X” appeared.
Subjective and Cardiovascular Effects
Participants completed Visual Analog Scale (VAS) forms,
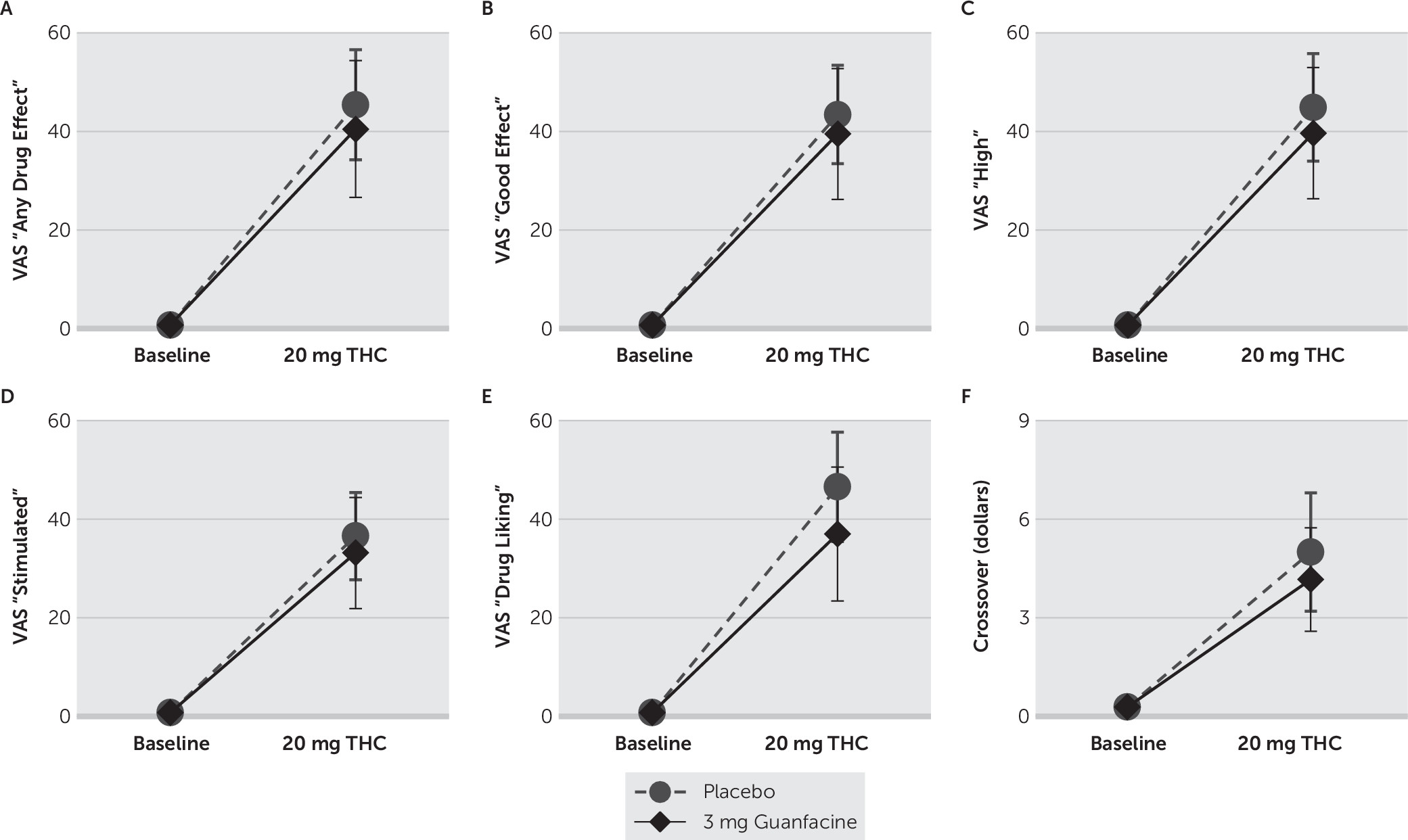
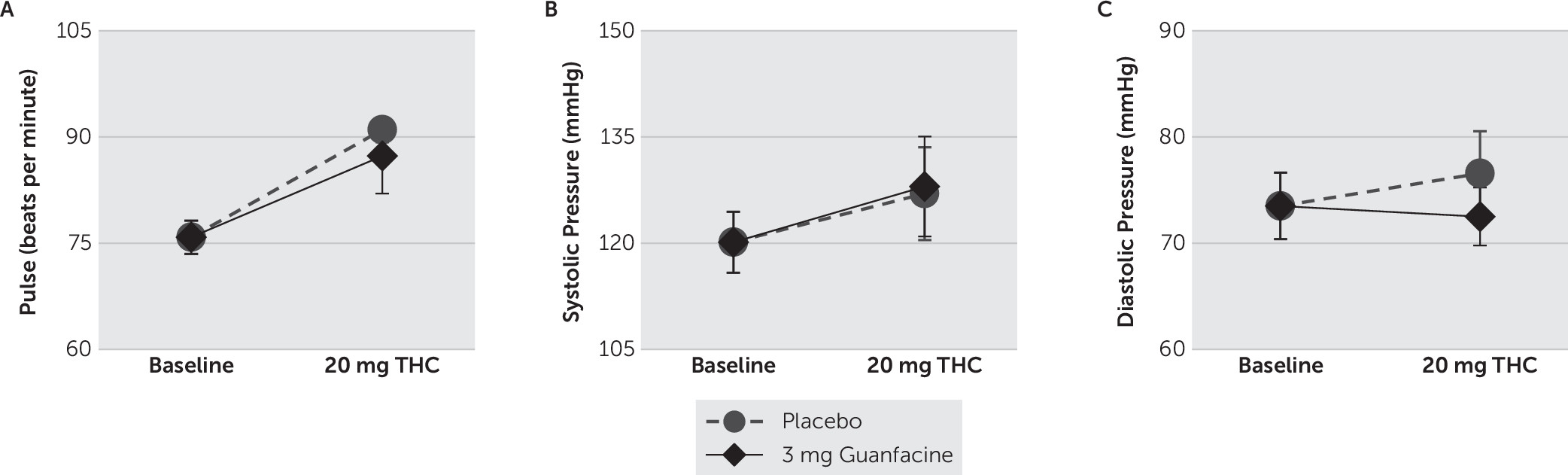
46–48 modified for cannabis, to rate the subjective effects produced by THC. VAS ratings, which were measured on a continuous scale digitized between 0 (not at all) and 100 (strongest ever), included ratings of “anxious,” “any drug effect,” “bad effects,” “depressed,” “desire (for cannabis),” “drug liking,” “good effects,” “high,” “likely to use (cannabis if accessible),” and “stimulated.” VAS ratings and cardiovascular measures (i.e., heart rate and blood pressure) were collected before and at 15-minute intervals for 1 hour after THC administrations.
Data Analyses
Cognitive data collected on day 1, and VAS and cardiovascular data collected 15 minutes before administration of the first THC dose on day 2, were averaged across the two treatment periods to establish a baseline for each participant before analyses. Data were subjected to repeated- measures analyses of variance (ANOVAs) with planned comparisons to contrast the effects of THC during treatment with placebo and guanfacine. To compare the means of each treatment on the effects produced by THC, we conducted pairwise multiple comparisons using Fisher’s least significant difference test.
For the HVLT, ANOVAs were calculated on 1) total recall (the total number of words recalled correctly on learning trials 1–3); 2) learning slope (the average number of new correct words recalled per trial); 3) delayed recall (the number of words correctly recalled on the delayed recall trial); and 4) percent retained (delayed-recall score divided by the higher score from trial 2 or 3). For the dual n-back task, ANOVAs were calculated on 1) auditory and 2) visual trial accuracies; 3) auditory and 4) visual trial reaction times (RTs); and 5) mean and 6) maximum n-levels achieved. For the CPT, ANOVAs were calculated on errors of 1) commission, 2) omission, and 3) perseveration, and 4) RT. For VAS ratings and cardiovascular data, ANOVAs were calculated on maximum or peak ratings and measures. Data are presented as means±standard deviations, except as displayed in graphic illustrations in which data are presented as means±standard errors. Cohen’s d effect sizes, to compare the means of each treatment on the effects produced by THC, are included for significant findings. Statistical significance was set at p<0.05, and analyses were conducted with SigmaPlot 12.0 software.
Discussion
This double-blind, placebo-controlled human laboratory study assessed the impact of guanfacine treatment on the cognitive, cardiovascular, and subjective effects produced by oral THC administration in volunteers who began using cannabis during adolescence. As was expected, THC (20 mg) alone decreased accuracy on working memory and attentional tasks and increased positive subjective ratings. The most consistent treatment effects were observed on working memory. Specifically, in comparison with treatment with placebo, treatment with 3 mg of guanfacine significantly reduced THC-induced adverse effects on spatial working memory performance.
We found that THC had a greater negative impact on spatial working memory than on attention or verbal recall. Indeed, cannabinoids have been reported to acutely impair spatial working memory in rodents,
49 healthy adults,
50 and adolescent cannabis users.
51 Furthermore, that spatial but not auditory working memory was affected in these adolescent-onset cannabis users is consistent with findings that acute THC administration impairs spatial working memory at doses that do not significantly impair object working memory in adolescent rhesus monkeys.
21 The adverse effect of THC on attention reported here is consistent with a large body of evidence for various, often dose-dependent, impairments in attention related to acute cannabinoid exposure.
52–59 On the other hand, the lack of effect of THC on HVLT performance is consistent with one report of minimal acute effects of cannabinoids in cannabis users
60 but not another.
54 Overall evidence regarding the acute effects of cannabinoids on certain cognitive domains has been mixed [reviewed by Broyd et al.
61]; however, a number of factors likely contribute to the inconsistent findings between studies, such as the different tasks used, and differences between study subjects, including frequency and quantity of cannabis use, and duration of abstinence. Of note, some data suggest that frequent cannabis users may be less sensitive to THC-induced impairment than are occasional smokers, despite achieving similar THC concentrations and subjective ratings.
62 This apparent tolerance to THC impairment may be a result of pharmacological adaptation
63 or behavioral compensatory mechanisms for deficits. For example, there is evidence that despite similar scores on cognitive tasks, cannabis smokers demonstrate significantly more brain activation and recruitment of alternative neural networks.
64–66 Our findings further indicate that acute adverse effects in chronic cannabis users may be underestimated and may require additional forms of assessment alongside traditional cognitive tests. Nonetheless, we found that both auditory and visual memory along with aspects of attention were more accurate during short-term treatment with guanfacine. Furthermore, beyond demonstrating statistical significance, the treatment effects presented here meet the threshold of a minimum clinically important difference (MCID) based on a distribution-based method involving effect size with a cut-off value of d=0.2.
67,68 On the basis of convention,
69 the effect sizes we report are considered moderate for both spatial and auditory working memory performance and are considered large for errors of omission. Because neither treatment-related changes to functional status nor laboratory-based assessments of real-world function were completed in this study, it is important to note that these observed changes still meet MCID criteria by a variety of effect sizes and merit further consideration.
The beneficial effects of guanfacine on spatial working memory and attention described here are consistent with a report that guanfacine (29 μg/kg) improved both spatial working memory and attention in healthy subjects.
30 Although the nonselective α2-adrenergic receptor agonist, lofexidine, has been reported to impair attention in cannabis users,
39 differential effects of α2-adrenergic receptor agonists on cognitive functions have been consistently reported in both humans
30,70 and monkeys.
71 These studies collectively suggest that the greater selectivity of guanfacine for the α2A receptor subtype underlies its potency for enhancing attention and working memory. Furthermore, although acute THC administration increased VAS subjective ratings in a manner consistent with previous studies,
72–76 no significant differences in ratings were observed between when THC was administered with either guanfacine or placebo. Thus, in contrast to other therapeutic agents such as modafinil, which has been associated with increases in anxiety, aggression, and negative subjective scores,
77–79 and lofexidine, which has been associated with increases in subjective ratings of “good drug effect” and “sedated” in cannabis users,
39 the beneficial effects of guanfacine on cognition reported here do not appear to be the result of secondary subjective effects.
As far as therapeutic targets are involved, most medications studied for treatment of CBUD have focused on managing withdrawal symptoms.
80 Indeed, these symptoms are common upon abrupt cessation of cannabis use and cause significant distress, thereby contributing to relapse.
81 On the other hand, behavioral therapies, such as cognitive-behavioral therapy, focus on building cognitive and emotional skills, to strengthen the ability of individuals to manage cravings, as well as to cope with withdrawal symptoms that might trigger drug use. Thinking of CBUD as a cluster of problems that reflect impaired cognition might be critical for addressing the persistent risk of relapse to cannabis use; 1-year abstinence rates range between 19% ανδ 29% across behavioral treatment studies.
82 Evidence that the adolescent PFC is particularly vulnerable to the adverse effects produced by cannabis,
16–19 coupled with the negative impact cognitive deficits have on treatment outcomes for CBUD,
4,5 supports targeting PFC-mediated cognitive functions to improve treatment outcomes. The potency of guanfacine to selectively improve cognitive functions dependent upon PFC function,
83,84 in both adolescent-onset cannabis users and abstinent cocaine-dependent individuals,
85 supports the potential utility of guanfacine for improving treatment outcomes by targeting cognitive functions in cannabis users.
37 Results from the current study provide preliminary support for the idea that by mitigating the acute adverse effects of THC on PFC-mediated cognitive abilities, guanfacine treatment might help prevent a lapse or a single incident of cannabis use from resulting in a relapse to sustained cannabis use.
There are several limitations of the current study. As the term of our study was relatively short and the sample of participants was small, the findings presented here will require further support. It is also important to note that we used a single (3 mg) dose of guanfacine, and ongoing study will be needed to determine optimal dosing and scheduling of medication delivery, especially because the beneficial effects of treatments on PFC-mediated cognitive functions display inverted, U-shaped dose-response curves.
86 Furthermore, it is unclear to what extent acute THC adverse effects or reversal of these effects contribute to more persistent cognitive changes and deficits, and this will be an important area for future investigation regarding the utility of medications such as guanfacine that target cognitive processes implicated in addiction. In addition, certain general limitations are associated with effective modeling of cannabis usage and outcomes in the community. Dronabinol, while well-suited to characterize the effects of THC, may not fully capture the heterogeneous neurocognitive effects of cannabis use, especially those that may be mediated by non-THC constituents of cannabis. For example, there is some evidence that cannabidiol, another major constituent of cannabis, may protect users from acute, THC-induced memory impairments.
87,88 Furthermore, in the present study, there was environment control for otherwise heavy patterns of cannabis use ranging from 2 to 20 times per day, and it is unclear whether the medication effect that was observed in this investigation would translate to more frequent patterns of use. Similarly, quantitative THC levels were not measured throughout this study, and there may be differences in dronabinol effect on measures of interest secondary to interindividual pharmacokinetic variability, especially given a range of cannabis use patterns prior to testing. Finally, although guanfacine was safely combined with THC in our study, and no unanticipated adverse effects were reported, further investigation is warranted to monitor for long-term adverse outcomes. Nonetheless, the findings presented here support that treatment with 3-mg guanfacine reduces acute THC-induced adverse effects on cognition in adolescent-onset, heavy cannabis users meeting CBUD criteria. Given concerning trends in cannabis use and CBUD, medications like guanfacine that stabilize PFC function and strengthen cognition merit further exploration, for minimizing acute adverse effects associated with THC intoxication, as initially demonstrated here, but perhaps also for mitigating the severity of relapse, enhancing cannabis treatment responses, and attenuating chronic processes that contribute to problematic use in adolescent-onset, heavy cannabis users.