Models of basal ganglia physiopathology are traditionally founded on sensorimotor functions of this circuit.
1 However, the role of the basal ganglia has been dramatically extended into nonmotor domains during the past decades.
2 The basal ganglia play a critical role in cognitive functions, such as language, attention, memory and executive function, and other nonmotor domains.
3,4 Indeed, disorders of the basal ganglia produce a combination of sensorimotor, autonomic, cognitive, and psychiatric symptoms.
5–12 The high incidence of cognitive deficits and/or neuropsychiatric symptoms in basal ganglia disorders reflects the multiple functions of the basal ganglia in these nonmotor domains.
8,9,13 An obvious example is Gilles-de-la-Tourette syndrome, which is both a neuropsychiatric disorder and a movement disorder. However, this is not an isolated case.
Most basal ganglia disorders have an impact on the three main categories of neuropsychiatric symptoms: cognition, emotion, and behavior. A majority of patients with either Parkinson’s disease, Huntington’s disease, Gilles-de-la-Tourette syndrome, supranuclear palsy, or corticobasal degeneration
14–18 exhibit neuropsychiatric symptoms. Ninety percent of parkinsonian patients and nearly all patients with Huntington’s disease (98%) exhibit at least one neuropsychiatric manifestation.
17,19 While most movement disorders, including Parkinson’s disease, involve extrabasal ganglia lesions, imaging studies have shown a correlation between basal ganglia lesions and cognitive as well as noncognitive neuropsychiatric symptoms.
20 Affective, apathetic and cognitive symptoms, including obsessive-compulsive disorder (OCD), suggest that basal ganglia lesions reduce the connectivity of the prefrontal and frontal cortex (connectivity disconnection syndrome), and thus lead to executive dysfunction.
21–23 Another key symptom of Parkinson’s disease, supranuclear palsy and Huntington’s disease is loss of motivation, which could be linked to pathological activity in the input nuclei (the striatum) and/or the output nuclei of the basal ganglia (the globus pallidus pars interna [GPi]).
24–26 Additionally, in parkinsonian patients, the incidence of neuropsychiatric symptoms correlates with cognitive deterioration.
19,27 About half of these patients suffer depression, sometimes as a prodromal manifestation, which is often associated with faster worsening of cognitive and motor functions.
28–30 Less frequently, anxiety can dominate the neuropsychiatric spectrum in parkinsonian patients, while a smaller fraction of this population develops psychosis with hallucinations and delusions.
19,31 Although a more detailed description is out of the scope of this review, it is worth noticing that basal ganglia dysfunction is also related to bipolar disorder, schizophrenia, mania, and delusion.
32–35Overview of Basal Ganglia Circuitry
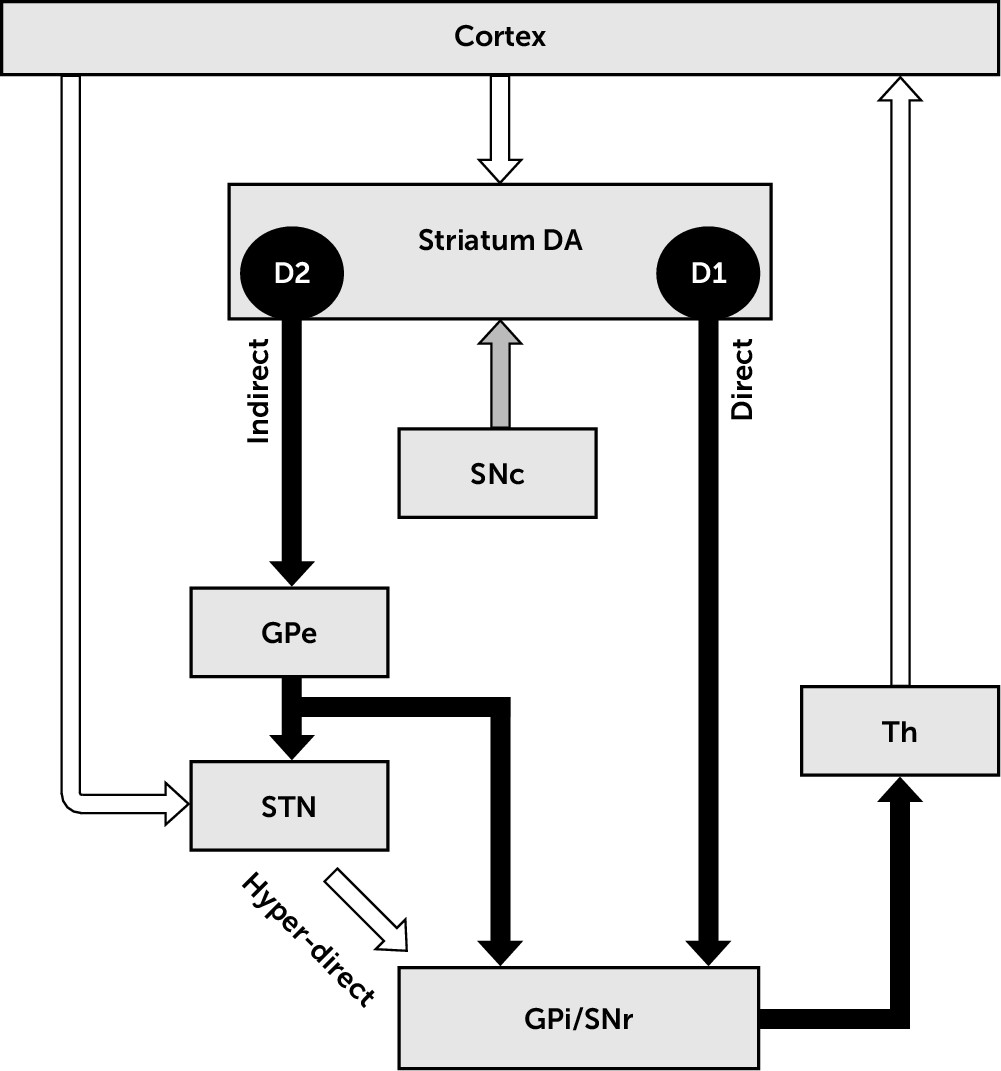
The basal ganglia (
Figure 1) are part of the cortico-subcortical circuits and the networks to which they contribute.
1,36 The striatum is the most prominent input nucleus to the basal ganglia and receives dense excitatory (glutamatergic) topographic projections from virtually all cortical areas. The striatum can be subdivided into a ventral part (nucleus accumbens), the dorsomedial striatum ([DMS] or caudate), and the dorsolateral striatum ([DLS] or putamen). The putamen receives sensorimotor and thalamic projections, while the nucleus accumbens and the caudate receive input from cognitive and limbic areas including those from the frontal cortex, prefrontal cortex, the insula, and temporal areas.
37 The subthalamic nucleus (STN) is a second input nucleus to the basal ganglia, which also receives topographic projections from most cortical areas.
38 The topographic organization of projections between nuclei is a critical anatomical feature of the cortico-basal ganglia-thalamo-cortical (Cx-BG-Th-Cx) circuits. An effect of this organization is the functional segregation of cortical downstream, although cross-talk between parallel pathways is also present.
39Regarding sensorimotor channels, somatotopic organization of cortical projections to the striatum and STN is maintained along Cx-BG-Th-Cx circuits.
39 These circuits are characterized by consecutive segments of inhibitory (GABAergic) and excitatory (glutamatergic) projections between nuclei, driving cortical neuronal activity downstream. Briefly, the cortical glutamatergic drive exerts excitatory control over striatal efferent neurons (medium spiny neurons [MSN]), which project to the basal ganglia output nuclei via two pathways.
40 The direct pathway is a GABAergic monosynaptic projection, while the indirect pathway is a GABAergic multisynaptic projection. At the level of the striatum, these two pathways are mostly segregated in two populations of MSN, expressing mostly different types of dopaminergic receptors.
41 The MSN of the direct pathway express D1 dopaminergic receptors, the activation of which by dopamine has a facilitatory effect on these neurons. In contrast, the MSN of the indirect pathway express D2 dopaminergic receptors, the activation of which by dopamine has an inhibitory effect on these neurons. In human and nonhuman primates, the output nuclei of the basal ganglia consist of the GPi and the pars substantia nigra reticulata (SNr). The GPi receives its major inputs from the striatum.
40 Additionally, the hyperdirect pathway bypasses the striatum, conveying excitatory impulses from the cortex to the globus pallidus through the STN.
42This anatomic-functional model stresses the importance of the balance of activity between the direct, indirect, and hyperdirect pathways on basal ganglia processing.
1 In conditions with lesion of the dopaminergic nigrostriatal pathway (i.e., parkinsonism), the model predicts that loss of dopamine results in an imbalance in favor of the indirect pathway, leading to decreased inhibition and increased neuronal firing activity in the output basal ganglia.
1,43 Secondarily, this imbalance leads to an inhibition of the thalamus and cortex.
44 Excitation and inhibition of the thalamus are traditionally considered to promote the selection and inhibition of movement, respectively; therefore, pathologic thalamic inhibition produces akinetic symptoms, according to this model.
1While traditional models of the basal ganglia have shown extraordinary resilience to time, anatomic details have gained spectacular complexity during the past decades.
37,45,46 Today, it must be acknowledged that the structure and function of the basal ganglia are more complicated than described in the aforementioned models. In a seminal review article in this journal, Mega and Cummings
47 described five parallel anatomic circuits linking frontal to subcortical regions in the human brain. Each circuit was named according to its cortical site of origin, and their descriptions included not only anatomic details but also their neurochemical modulation and their clinical correlates. In
Table 1, we replicate this approach, updating the information as needed. The following paragraph introduces some of the most relevant new information about these frontal-subcortical circuits.
The striatal microcircuitry, once considered as a simple structure converging cortical input on deep nuclei, is now viewed as a complex network processing efference from not only the cortex and the basal ganglia but also from extrabasal ganglia origins, and that includes multiple interneuron types.
48–52 GABAergic interneurons play an important role in the striatum. While a subpopulation of GABAergic interneurons exerts their inhibition on a short distance, another subpopulation bridges distant striatal territories.
53–55 These newly discovered anatomic details underlie complex processing of motor and cognitive information in this major input center of the basal ganglia circuit.
49 Furthermore, the density of gap junctions that interconnect striatal interneurons, also connecting them electrically to MSN, underscores the scale of integration of cortical drive across different functional territories of the striatum.
46 In addition, anatomical and pharmaco-biochemical studies have accumulated a large body of evidence showing that glutamatergic, GABAergic, and dopaminergic interactions are insufficient to characterize neuromodulation in the striatum.
50,51The basal ganglia are not an isolated set of nuclei. They receive indolaminergic, catecholaminergic, and acetylcholinergic projections from extrabasal ganglia regions, all of which contribute to modulation of the activity of basal ganglia neurons and interneurons. In the case of Parkinson’s disease, loss of neurons in the pedunculopontine nucleus (PPN),
56 the locus coeruleus,
57–60 and the substantia nigra pars compacta
57–60 is characteristic and contributes to both motor and neuropsychiatric symptoms.
The dorsal raphe nucleus (DRN) sends serotoninergic projections to the basal ganglia including the striatum; the globus pallidus; the substantia nigra; and, to a lower extent, the STN. Serotoninergic receptors are expressed all along the Cx-BG-Th-Cx circuits, if with some heterogeneity. The decrease in serotonin levels in the basal ganglia is well documented both in postmortem studies on parkinsonian patients
61,62 and in animal models.
62,63 Clinically, the alteration of serotoninergic neurotransmission contributes to the high incidence of depression in Parkinson’s disease.
64The PPN projects acetylcholinergic fibers diffusely throughout the Cx-BG-Th-Cx circuits (striatum,
65,66 STN,
65–74 substantia nigra mostly pars compacta,
65,66,75 globus pallidus
65,66,71 including external
66,73 and internal segments,
66,73 thalamus
66,71,73,76,77 including the central medial-parafascicular complex,
73,74 and primary motor cortex
74), in addition to brainstem nuclei, cerebellum, hypothalamus, and spinal cord.
78 While the PPN has been considered mostly involved in the regulation of locomotion and sleep, there is an accumulation of evidence favoring its role in action-reward prediction, learning, attention and decision making.
79The locus coeruleus (A6) projects noradrenergic fibers to the Cx-BG-Th-Cx circuits, including the intralaminar complex of the thalamus and the cortex as well as the brainstem nuclei, cerebellum, hypothalamus, and spinal cord.
80 Aberrancy of noradrenergic neurotransmission to these circuits may contribute to depressive symptoms related to parkinsonism, but the modulation of basal ganglia functions by the locus coeruleus remains poorly understood.
81Several neuroactive peptides modulate the Cx-BG-Th-Cx circuits, including tachykinins, enkephalins, dynorphin, somatostatin, and neuropeptide Y, all of which are used by distinct subsets of basal ganglia neurons.
82 Moreover, the function and structure of higher cortical areas, such as the insula and other areas of cortex, are also affected by functional and structural abnormalities of the frontal-subcortical circuits, and their secondary dysfunction has the potential to further contribute to neurobehavioral symptoms among persons with disorders affecting the basal ganglia.
60,83The basal ganglia are complex architecturally, neurochemically, and with respect to their structural and functional connectivity. The simplified, traditional structural-functional model of the basal ganglia has provided a useful heuristic for research and education.
1 However, it is naïve anatomically and functionally, particularly with respect to its reliance on linear dynamics in explorations of the pathophysiology of basal ganglia disorders. Developing new pharmacological and procedural approaches that more effectively treat basal ganglia disorders requires new heuristics that acknowledges and incorporates more fully the complexity of these structures and the circuits and networks to which they contribute.
Rate-Based Model of the Basal Ganglia
The description of summed excitatory/inhibitory drives onto the output nuclei of the basal ganglia provides a framework for considering firing rate as the coding scheme of neural information in the Cx-BG-Th-Cx circuits. In neuronal systems, a rate code assumes proportionality between the firing rate of neurons and some environmental clue or input signal. If this proportionality holds, the state of the neuronal system can be characterized by the central tendency of the firing rate, defined over a given time window.
84,85 A trait of such a rate code is its robustness to noise, since information is carried by the central tendency and not by the precise timing of single spikes.
Evidence in favor of a rate code in the basal ganglia links striatal dopamine function to firing rate and motor activity as well as nonmotor activity, in normal and pathological conditions.
86–89 In the nonmotor domain, a rate code plays a role in aversion learning and reward encoding in the ventral pallidum, probably accompanied by a population code.
90,91 In the sensorimotor system, a rate code is supported by the negative correlation between the rate of discharge of the GPi and specific features of motor activity, like movement onset and velocity.
92 In parkinsonism, two main categories of clinical and experimental studies support the rate hypothesis: 1) studies reporting higher firing rate in the GPi of parkinsonian subjects and 2) studies reporting that prodopaminergic therapies reduce the GPi firing rate.
93–97 Extensive discussion about these studies can be found in the literature.
1,98,99On the other hand, a large body of experimental and clinical evidence contradicts the rate-based model at every level of the Cx-BG-Th-Cx circuit. In the primate GPi, the predicted proportionality between firing rate and severity of parkinsonian symptoms is absent, and hypokinetic symptoms can be observed without any increased firing rate.
100 Direct measurements from the human STN also contradict the rate hypothesis: the firing rate in the STN of parkinsonian and epileptic patients without movement disorders is similar.
101 In the striatum, MSN show local hypermetabolism and higher firing rate in Parkinson’s disease, but prodopaminergic treatments further increase their firing rate, contradicting the predictions of the rate-based model once again.
102,103 At the cortical level, parkinsonian symptoms can occur without change in the mean spontaneous discharge rate of neurons.
104 Finally, the rate hypothesis fails to explain the benefits of deep brain stimulation (DBS) across the spectrum of movement disorders.
105–107 This growing body of controversies around the rate-based model of the basal ganglia has weakened the hypothesis of rate coding in the basal ganglia.
108–110Oscillatory Model of the Basal Ganglia
The second main hypothesis of processing of information in the basal ganglia is based on oscillatory phenomena, which can be identified both in single neurons and network activity (local field potentials [LFP]) along the Cx-BG-Th-Cx circuit.
100–104 Oscillatory activity has been considered a coding scheme in some work, but more often it is regarded as an emergent property.
1,115–118 In movement disorders, experimental and clinical measurements have reported positive correlations between β-band power (10–30 Hz) and hypokinetic conditions,
1,113,119–131 as well as positive correlations between γ-band power (over 40 Hz) and motor activity.
132–136Regarding parkinsonian symptoms, β-band power in the basal ganglia correlates with tremor,
137 rigidity,
138 bradykinesia,
139 and freezing of gait.
140,141 As could be expected, oscillations in the basal ganglia correlate not only with motor function/dysfunction but also with neuropsychiatric symptoms.
114 In the α-band (8–12 Hz), oscillations are influenced by emotional stimuli and depend on the affective state, being altered in depressed patients with Parkinson’s disease.
142 Impulse control disorders have characteristic oscillations in the theta-alpha band (4–10 Hz) in the STN in patients with Parkinson’s disease.
143 Theta and beta power in the STN are also related to decision making and conflict evaluation.
144,145 Oscillations in the β-band in the cortex and the basal ganglia are associated with behavioral manifestations, such as behavioral stopping, semantic encoding, and memory.
117,146,147 Finally, γ-band activity is related to cognitive processing.
148,149Although oscillatory activity has been identified over a broad spectrum of frequencies in the basal ganglia, the oscillatory hypothesis focuses mostly on two frequency bands, the β-band and the γ-band, resembling the notion of two opposite control subcircuits of the rate-based model: prokinetic and akinetic. However, evidence is contradictory with respect to this hypothesis. The relation between high β-band power and hypokinetic states is challenged by studies comparing LFP in different movement disorders.
150–153 The proportionality between β-band power and the severity of symptoms remains debated as well.
100 In the cortex, findings are also contradictory: The effect of L-DOPA on β-band activity reports either no change, increased activity, decreased activity, or mixed effects.
154,155 Concerning the γ-band, although γ-band activity has been reported as stronger in the parkinsonian state, γ-band power can be further increased by antiparkinsonian treatments.
155,156Adaptive mechanisms have been proposed to explain these discrepancies and integrate these studies into the framework of the oscillatory hypothesis.
157 In the Cx-BG-Th-Cx loop, the increase in γ-band activity was suggested as a prokinetic adaptive mechanism to oppose the putative antikinetic effects of the pathological increase in β-band activity.
156 At a larger scale, the increased connectivity in the cerebello-thalamo-cortical loops is envisaged as a prokinetic mechanism to compensate the dysfunctional Cx-BG-Th-Cx loops.
158The anatomical origin and mechanisms of propagation of oscillatory activity across the frontal-subcortical circuit remain unclear, in health and in Parkinson’s disease.
159 Indeed, a unifying hypothesis of the causal relation between oscillatory power at different frequency bands and clinical manifestations of disease remains undeveloped. The challenge to its development is the multiplicity of the phenomena for which it needs to account, including attention processing, periodic sampling of the state of the periphery, increased response inhibition, and mechanisms to maintain the neurobehavioral and motoric status quo.
1,119,120,147,160–163Pitfalls of the Rate and Oscillatory Models
The preceding review suggests that the pathophysiology of the basal ganglia is associated with alterations in the neuronal firing rate and oscillatory activity
98,110,164,165 but neither model—both of which are predicated on linear measures of single unit activity and/or LFP—provides satisfactory accounts of Cx-BG-Th-Cx circuit function and dysfunction in health and disease. In particular, Parkinson’s disease, the classic basal ganglia disorder, challenges the utility of these models.
Critics of linear dynamic models of the basal ganglia have pointed out that Parkinson’s disease may be understood as a breakdown of information processing in cortico-subcortical circuits with a wide range of motor symptoms (including tremor, rigidity, bradykinesia, and hypokinesia), autonomic dysfunctions (orthostatic hypotension, altered heart rate variability), sleep disturbance, pain, and cognitive and neuropsychiatric manifestations.
166 In the sensorimotor domain, neither a change in firing rate nor a change in oscillatory activity can at once explain rigidity, difficulty initiating movement, rhythmic muscle contractions (i.e., tremor), and problems with integrative motor function (i.e., coordination). Similarly, these models also cannot account for the concurrent manifestation of the broad range of nonmotor symptoms—including cognitive, emotional, behavioral, and autonomic symptoms, both hypokinetic and hyperkinetic—produced by Parkinson’s disease. As mentioned by Montgomery,
167 the rate and oscillatory hypotheses attempt to account for such symptoms by dichotomizing basal ganglia control mechanisms into two opposing stable states (i.e., “on” and “off”). Therefore, both hypotheses fail for the same reason as explanatory models of basal ganglia pathophysiology: They are unable to account for the complex and inherently mixed nature of parkinsonian symptoms as well as their responses to treatment.
167During the past two decades, new mathematical frameworks have been introduced in the field to characterize the dynamics of the basal ganglia in normal and pathological conditions. These new frameworks show that central tendency measures (e.g., firing rate or frequency power)—and linear features, more generally—cannot fully characterize the dynamics of physiological signals in the Cx-BG-Th-Cx circuits.
168–170 Conversely, mathematical methods developed in line with Shannon’s
171 theory of information allow the quantification of features related to information transmission in complex systems. An analysis of information content in the Cx-BG-Th-Cx circuits might provide new models to describe the whole spectrum of basal ganglia disorders, as opposed to classic models, which force a classification of movement disorders in two categories depending only on the amount of motor activity produced (hypo- and hyperkinetic disorders).
167 Additionally, nonlinear analysis methods provide alternative approaches to investigate the effects of treatments that cannot be reduced to either gross excitation or gross inhibition, such as DBS or new therapies targeting the catecholaminergic (i.e., adrenergic) or indolaminergic (e.g., serotoninergic) pathways.
87,165,172–175From studies using nonlinear methods of analysis, the temporal structure of spike trains reveals patterns that are characteristic of the activity of healthy and diseased basal ganglia.
176 The presence of these patterns implies that: 1) a rate code cannot fully account for the transmission/processing of information in the basal ganglia; and 2) symptoms of Cx-BG-Th-Cx circuit dysfunction are related to a breakdown of temporal organization in the spiking activity of the basal ganglia.
85,177Conceptualization of Basal Ganglia Function Based on Nonlinear Dynamics
The term
nonlinear system refers to organized systems generating an output that is not linearly proportional to the system’s input. Nonlinear systems exhibit complex behavior, which cannot be fully described by a linear combination of the individual behavior of their constituent parts.
178 While linear dynamic systems are fully predictable (consider the distance that a car travels in 1 hr at 50 km/hour), and stochastic systems are fully unpredictable (consider the lottery), nonlinear systems may exhibit complexity and sensitivity to initial conditions and, hence, limited predictability.
179 A historically relevant example in the field of nonlinear dynamics is the weather forecast: while short-term predictions are robust, long-term predictions remain uncertain.
180,181 The complex temporal output of nonlinear systems results from the activity of multiple, sometimes parallel or nested, control loops, acting at different time scales.
179,181,182In the basal ganglia, such loops have been identified at the microcircuitry
183,184 and macrocircuitry levels.
2,86,185,186 The resultant irregularity observed in the signal is not stochastic, but chaotic (see
Figure 2). Chaoticity (i.e., the extent to which something is chaotic) can be measured from time series with diverse methods and is directly proportional to the rate of information loss (i.e., entropy) and inversely proportional to predictability.
182 While linear methods of analysis focus on central tendencies to describe the state of a system in time and/or frequency domains, nonlinear methods measure the persistence of certain time patterns or “shift” in the irregularity of the time series (related to entropy) and uncover hidden, complex patterns of temporal organization.
Normal and pathologic human behaviors are manifestations of the complexity of the nervous system.
187,188 This complexity is reflected in brain activity at every scale and can be measured by a variety of methods, including spike trains (i.e., time-series electrical signals) recorded from individual neurons in the brain, electroencephalographic and magnetoencephalographic techniques (EEG and MEG, respectively), and functional magnetic resonance imaging (fMRI).
189–192 Use of these methods to describe normal complexity in brain activity is essential to understanding and treating changes in the complexity of brain activity wrought by disease, including mental illness.
In a thought-provoking paper, Yang and Tsai
193 proposed that mental illness is related to the loss of brain complexity and that it can therefore be measured with complex dynamics’ tools. The foundation for this proposal is evidence of modifications of normal brain complexity in schizophrenia, anxiety disorders, dementias, autistic spectrum conditions, attention deficit/hyperactivity disorder, and sleep disorders,
194–204 in which contexts, alterations in the complexity of brain activity are related to cognitive and emotional symptoms, may provide biomarkers of disease, and may be remediated by pharmacotherapies.
205 In Alzheimer’s disease, cognitive deterioration correlates with a reduction of complexity of brain signals.
200,206–208 Alterations of the complexity of brain activity are associated with depressive symptoms, including reduced complexity of the EEG in patients with depression,
209,210 increased complexity of the MEG in younger patients with depression,
195 and increased complexity in the EEG in patients with chronic stress.
211 Reduction of MEG-determined complexity correlated with pharmacologically-induced remission of depressive symptoms.
195 In summary, a healthy psyche is related to a certain degree of age-dependent complexity in brain activity,
212 which can be measured with nonlinear methods from different invasive, as well as noninvasive, recordings of neurophysiologic signals.
In the case of the basal ganglia, a growing body of evidence shows that irregularity in physiological signals is not random in nature but exhibits complex, nonlinear temporal organization in neuronal firing activity,
195,213–217 LFP/EEG,
218–223 electromiography
224,225 and movement kinetics.
226–229 Stam et al.
230 identified nonlinear features in the EEG recorded from parkinsonian patients.
230 Later, entropy of EEG was reported to be higher in parkinsonian patients compared with healthy controls.
231,232 This finding was confirmed by Han et al., who further indicated that Parkinson’s disease is associated with increased complexity of the EEG’s rhythm.
233 Hohlefeld et al.
234 reported a positive correlation between long-range temporal correlations ([LRTC] a measure of temporal organization over different time scales) in EEG recordings and deep LFP from parkinsonian patients, supporting that complex patterns can be repeated across time scales ranging from milliseconds to tens of seconds.
234 Further studies have suggested that LRTC is a potential biomarker of pathological processing in basal ganglia disorders.
235,236 In Parkinson’s disease, chaoticity in the activity of GPi neurons diminishes as the severity of motor symptoms increases.
237 However, since normal brain complexity also diminishes with age, these effects could well depend on age or cognitive decline, a phenomenon that has not been studied yet.
238 Using a neural network model, Li and Sikström
239 modeled aging-dependent reduction of dopaminergic modulation by reducing neuronal nonlinearity, suggesting a mechanism that could be shared by normal aging and neurodegeneration.
239At the neuronal level, nonlinear temporal organization has been identified in interspike intervals (ISI) recorded from basal ganglia neurons in rodents, primates, and patients with movement disorders.
234–237,240–242 The irregularity of ISI from basal ganglia neurons results from the replication of complex patterns, which cannot be statistically explained from the probability distribution around a central tendency.
108,170,177, 243,244 Neurons in the striatum code reward of actions and integrate reward into movement, leading to action selection in specific social situations and participation in reward-based learning.
245,246 As is the case with motor symptoms, complex electrophysiological manifestations accompany neurobehavioral manifestations of basal ganglia dysfunction.
247,248 In light of the evidence, it is clear that any linear model is inadequate to characterize the irregularity produced by the basal ganglia and the information they convey.
169,249 The notion of a complex “neuronal language,” which has emerged from direct observations on the temporal organization of ISI from basal ganglia neurons, can help in building a new conceptual framework to study the pathophysiology of this circuit.
85,170,182,237,250Entropy Hypothesis
The term entropy comes from the field of thermodynamics, where it is related to the number of possible states of a system. Applied to the analysis of spike trains, entropy measures temporal irregularity. Statistical quantities, such as the standard deviation (SD) or the coefficient of variation (CV), measure dispersion of a signal from its central tendency, independently from the temporal order of the data. Something similar happens with frequential analyses, where the temporal domain is lost (e.g., the Fourier spectrum).
By contrast, the order of events in the time series is the crucial factor for the calculation of entropy, as well as other nonlinear measures (temporal structure, LRTC, among others). The value of entropy increases when an observed pattern is not followed by similar patterns in a time series. A time series that is highly predictable has relatively low entropy; a less predictable, more irregular process has higher entropy.
For example, consider the following sequences: A=[2 2 2 1 2 1 1 1 1 2], and B=[1 2 1 2 1 2 1 2 1 2]. Simple statistical measures are equal for both data streams: mean(A)=mean(B)=1.50, SD(A)=SD(B)=0.53, and CV(A)=CV(B)=0.35. However, entropy is higher in the first case; i.e., the more irregular time series (A). The following definitions apply, where x is the independent variable and n is the number of data samples:
Approximate entropy algorithm, m and r fixed at 2 and 0.1, respectively: ApEn(A)=0.51 and ApEn(B)=0.01.
Since linear measures are insufficient to fully characterize the complex nature of neural signals, entropy, among other nonlinear quantities, needs to be incorporated to describe the dynamics of the basal ganglia.
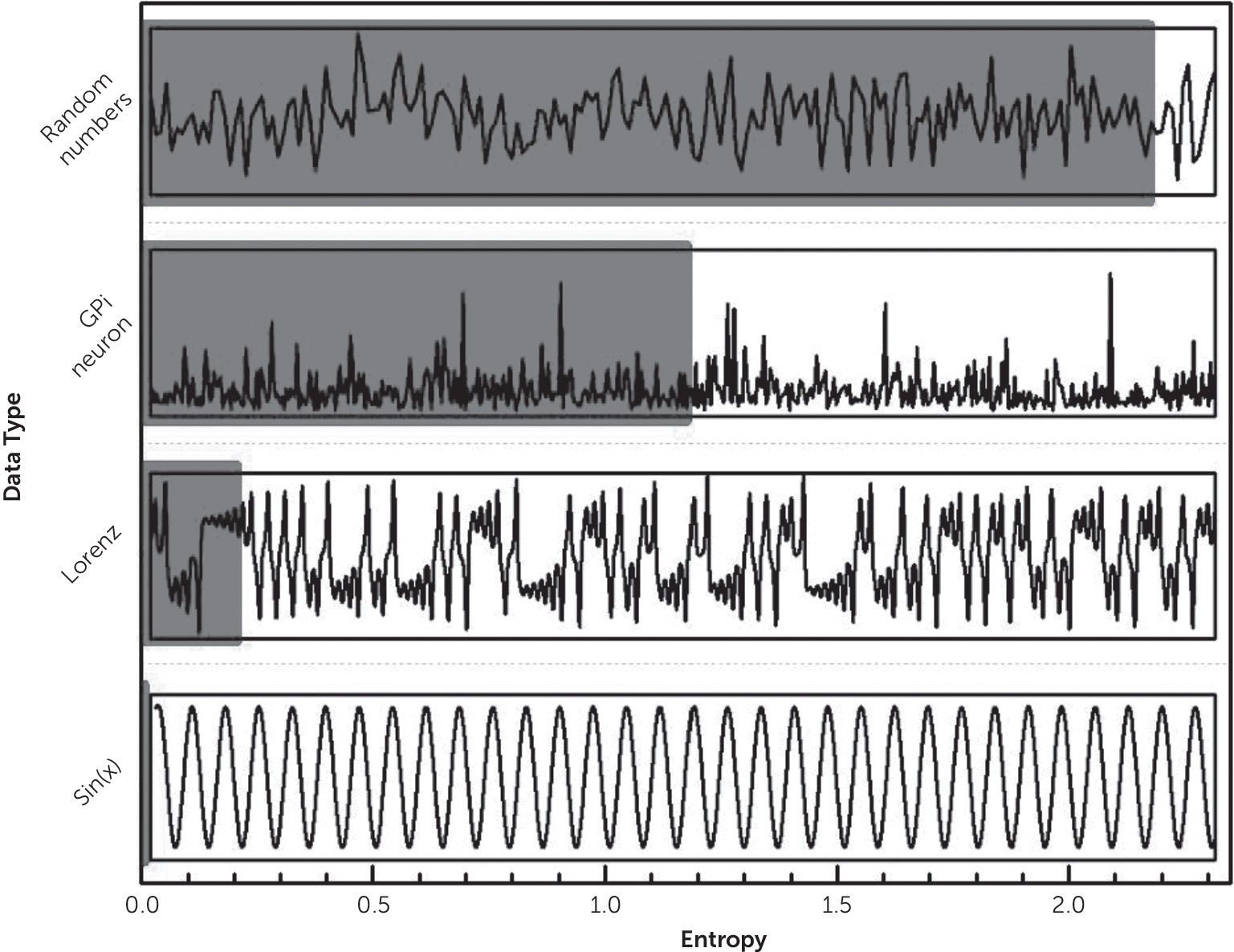
170,240,241,243,251,252 Figure 2 presents an example of a time series produced by a random number generator; a GPi neuron; a nonlinear system (Lorenz attractor); and a fully periodic system, sin(x), with their respective entropy values. The irregularity of ISI stands out as a striking feature of neuronal activity in the basal ganglia. This is similar in both normal and pathologic conditions. In the example shown, the entropy of a typical “parkinsonian” GPi neuron is above that measured from the low-dimensional nonlinear system but below the entropy of a stochastic system (a random number generator). As we discussed earlier, while linear models are unable to explain cognitive and behavioral symptomatology of basal ganglia disease, complexity measures show great potential as hallmarks of mental illness. Our proposal is that the deficiencies observed with linear models with respect to the motor, cognitive, emotional, and behavioral manifestations of frontal-subcortical circuit disorders can be addressed with nonlinear techniques. Hyperkinetic and akinetic motor disturbances are related to alterations in the complexity of activity in the frontal-subcortical circuits: neuronal entropy seems to be directly related to akinetic disorders and inversely so to hyperkinetic disorders.
250 How to reconcile excesses and deficits of cognition, emotion, and behavior associated with disturbances of these circuits and nonlinear measures is a promising field of research for the next years.
Experimental and clinical investigations show that dopamine modulates neuronal entropy in the basal ganglia. In Sprague-Dawley rats, lesions of the nigrostriatal pathway modify the relation between alertness and entropy at the entopeduncular nucleus (EPN, equivalent to the GPi in primates).
242,253 In this species’ EPN, the effect of dopamine depletion is a simultaneous increase of neuronal entropy and firing rate, plus an altered relationship between both.
254 Specifically, under nigrostriatal lesion, neurons with high firing rate in the output basal ganglia fail to downregulate entropy, offering a functional hallmark of chronic dopamine depletion.
244 Importantly, antiparkinsonian treatment (apomorphine, as well as DBS) reduces basal ganglia entropy.
251,252 Therefore, temporal organization of spike trains needs to be investigated as a hidden feature behind the effects of antiparkinsonian drugs. In contrast to the rate and oscillatory hypotheses, the entropy hypothesis supports the idea that irregularity in the time series of ISI is the result of dynamic processing of information within the basal ganglia. The conceptual weight of the model is shifted from anatomical connectivity to information processing.