Olfactory reference syndrome (ORS) is characterized by the core false belief that one emits a foul or unpleasant body odor.
1 Although ORS has been described for a century and across cultures,
2 no studies of olfactory function in individuals with this diagnostically challenging disorder have been reported. In the present case report, we characterize the olfactory function of a patient with ORS at baseline and after a short, self-administered course of olanzapine. Our findings empirically support the observation that odor stimuli in the environment can trigger parosmia and that concurrent parosmic perception of the malodor compromises the patient’s olfactory function at baseline. Furthermore, we found that olanzapine treatment, which produces improvements in parosmia, was accompanied by an improvement in the patient’s olfactory function. These novel findings have implications for how clinicians might think about the impact of ORS and its treatment on olfactory function and the ongoing debate regarding the classification and differential diagnosis of this complex and debilitating set of clinical features.
2Case Report
“Mr. A,” a 69-year-old man with a lifelong history of depression and chronic hepatitis C, presented with the chief complaint of the emission of a foul body odor for the past 5 years. The patient indicated that this stereotyped “rotten fish” perception was only evoked by smelling (i.e., it was not present when he was breathing ambient air). The patient did not recollect having an upper respiratory tract infection prior to the onset of parosmia. His daily functioning was significantly impaired due to the parosmia (e.g., he avoided social situations because of shame and fear of judgment). He also reported delusions of reference, such as the belief that people “cover their nose” and “recoil in disgust” when near him. In order to cope with the parosmia, he engaged in ORS-related repetitive behaviors, such as washing his hands and sniffing at and changing his clothes. This social avoidance and repetitive behavioral repertoire is consistent with features reported in other case reports of ORS.
3–5The patient received a score of 27 on the Yale-Brown Obsessive Compulsive Scale Modified for ORS,
5 indicating ORS with moderate severity. In the course of a week, he spent 3–8 hours a day obsessing over the parosmia. He had a history of intravenous drug abuse, and in 1992, he was diagnosed with hepatitis C viral infection. He last used intravenous drugs 29 years prior to participating in our smell testing. He presently receives hydroxyzine (25 mg) and orally ingests medical marijuana (1/3 g in oil form) once or twice a week to “calm his nerves.” He is not being regularly followed by a psychotherapist.
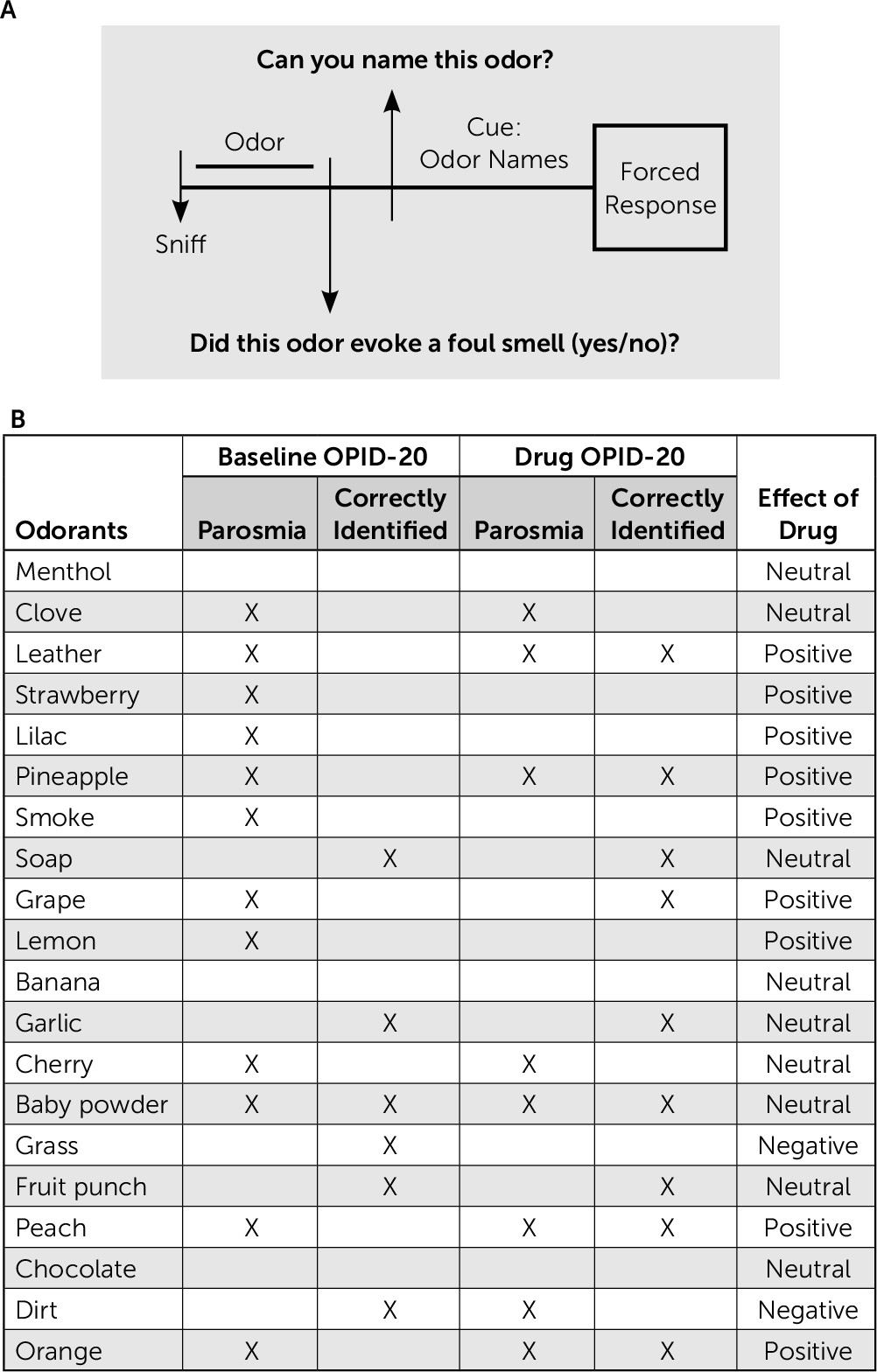
To examine the patient’s ability to identify odors, we administered an abridged version of the POEM [Percepts of Odor Episodic Memory] olfactory battery,
6 which was modified to include a question asking whether the odor presented evoked a parosmic perception (see
Figure 1A). At baseline, the patient reported that 60% of the 20 different odors presented (N=12) elicited a parosmic perception (see
Figure 1B). He exhibited a significant odor percept identification deficit compared with clinically normal age-matched controls, correctly identifying only 30% of the presented odors, compared with the age-matched cognitively normal individuals, who were able to identify 76% of odors presented on average.
6 To look for an interaction between the parosmia and odor identification, we recalculated the patient’s odor identification accuracy for odors that did/did not evoke the perception of a foul body odor. Among the 12 odors that evoked the perception of a foul body odor, the patient was only able to correctly identify one odor (8.33%). Among the nonparosmic odors (N=8), he correctly identified five odors (63%).
The patient scored in the normal range on the Boston Naming Test (29/30 correct), and he scored in the low-normal range on the Blessed Dementia Scale (31/37 correct).
One week after the baseline test, which included an acute, 3-day, low-dose course of olanzapine (2.5 mg days 5–7), the patient was retested with an identical olfactory battery. Olanzapine, which has been used in cases of ORS,
7–9 was reported by the patient to reduce parosmia in the past. In our odor-evoked experimental setting, it was similarly effective. Following treatment with the drug, the patient only found 40% of the odors (N=8) parosmic (see
Figure 1B). His overall odor identification accuracy on the Odor Percept Identification-20 test improved from 30% to 45%. Interestingly, he showed a higher degree of accuracy in naming parosmic (63%, 5/8) versus nonparosmic (33%, 4/12) odors. All of the parosmic odors (except one) identified post-treatment were the same as those identified pre-treatment. Of the seven parosmic odors, unchanged by the drug condition, 14% were identified correctly (N=1) at baseline (pre-treatment), whereas 71% were identified correctly in the post-treatment condition (N=5).
Overall, olanzapine positively affected the patient’s response to nine of the 20 odors presented during the odor identification test. Four odors (leather, orange, pineapple, and peach) incorrectly identified at baseline were correctly identified in the drug condition. Four odors (strawberry, lilac, smoke, and lemon) that elicited parosmia at baseline no longer elicited parosmia in the drug condition. The odor of grape, which was both incorrectly identified and elicited parosmia at baseline, was correctly identified and nonparosmic in the drug condition.
Olanzapine negatively affected two of the 20 odors presented during the odor identification test. The odor for dirt was the only odor of the 20 presented that was newly parosmic in the drug condition. It was also identified incorrectly in the drug condition, while it had been correctly identified at baseline. The odor for grass was identified incorrectly in the drug condition, while it was correctly identified at baseline.
The patient’s response (parosmia and odor identification) to the remaining nine odors (menthol, clove, soap, banana, garlic, cherry, baby powder, fruit punch, and chocolate) was unchanged by the drug.
Discussion
Our patient, who had been suffering from ORS symptoms for more than 5 years, showed a marked odor identification deficit, compared with age-matched controls, and a high frequency of odor-evoked parosmia. The odor identification deficit is unlikely due to a global naming deficit or the presence of dementia. At baseline, the patient named nonparosmic odors with greater accuracy, suggesting that evoked parosmia negatively affected his naming of parosmic odors. Given this result, we speculated that the neural basis of the parosmia associated with ORS may resemble instances of parosmia reported in temporal lobe epilepsy (TLE) that are associated with the perception of a noxious odor. In these TLE cases, the hyperexcitability of the cortex results in deficits in higher olfactory processing.
10Based on this model, we hypothesized that a drug that reduces parosmia would afford beneficial effects in olfactory processing, reflected as improved accuracy of odor identification. The patient reported that he had used a low dose of olanzapine acutely in the past to reduce the foul-odor perception triggered by his parosmia. In the setting of olfactory testing after the olanzapine course, we confirmed that fewer odors evoked parosmia, and we found an improvement in the patient’s performance on the odor naming task. Surprisingly, odors that no longer evoked parosmia were not more likely to be accurately identified. Rather, improvements in identification in the drug condition were more marked among odors that continued to evoke parosmia. This dissociation of odors with improved identification and improved parosmia, relative to the olanzapine-free testing, may be due to divergent effects of olanzapine on the generation of hedonic percept of parosmia and the semantic processing of parosmic odors.
The complex pharmacology of olanzapine includes antagonism of the serotonin (5-HT
2A, 5-HT
3, and 5-HT
6) and cholinergic (M2) receptors. We speculate that one or more of these activities could account for the clinical improvement witnessed in our patient. The 5-HT
6 receptor is highly expressed in the olfactory tubercle in the rat brain.
11 The olfactory tubercle is highly interconnected across brain regions and has been implicated as a site of integration across olfactory, visual, and auditory sensory input as part of the olfactory cortex,
12 as well as a candidate region for associating odor stimuli with hedonic and motivated behavior as part of the straitum.
13 Additionally, it receives input from the hippocampus and reciprocally communicates with the amygdala, thalamus, hypothalamus, and brainstem. Although its specific role in odor processing is still being characterized, neurons in this region do show odor-evoked activity (which increases when an odor is attended to), as well as odor-selective activity (which may be relevant for odor discrimination).
12It is likely that 5-HT
6 is expressed in the olfactory tubercle as a population of heteroreceptors on GABAergic and glutamatergic neurons, since it is in the hippocampus and the cortex.
11 This positions the 5-HT
6 receptor, and its antagonist olanzapine, as a potential regulator of the balance of excitatory-inhibitory signaling in the region (e.g., signal-to-noise ratio), with consequences for a higher-order olfactory processing task (e.g., odor identification). Indeed, electrophysiological characterization of the intrinsic properties of olfactory tubercle neurons suggests that the region contains glutamatergic and GABAergic networks.
12 The mechanistic implications of this antagonism for the observed reduction of parosmia (in relation to a role for this region in odor hedonics) are intriguing and hereto unexplored.
Olanzapine’s high-affinity antagonism of the 5-HT
2A receptor also may modulate GABAergic input sent to the hippocampus from the medial septum/diagonal band of Broca (MSDB). MSDB neurons can be activated in rat brain slices by bath application of serotonin (through the activation of the 5-HT
2A receptor) and inhibited by application of atypical antipsychotics such as olanzapine, suggesting that a population of septohippocampal neurons may be a target for the action of this class of drugs.
14 A disinhibition of GABAergic septohippocampal input to pyramidal neurons in the hippocampus, in a low-dose drug condition, may enhance odor naming and memory in an odor identification task.
15An effect on cholinergic neuromodulation by olanzapine could have relevant implications for the coding of odor representations and aspects of olfactory learning and plasticity. Cholinesterase inhibitors have been shown to augment muscarinic cholinergic neurotransmission in the brain.
16,17 A feedback loop has been described between cholinergic afferents from the diagonal band of Broca (HDB) in the Ch3 nucleus of the basal forebrain, the olfactory bulb, and the piriform cortex.
18 The granule and glomerular layers of the olfactory bulb receive extensive cholinergic input from the HDB.
19,20 Microdialysis studies show increased levels of extracellular acetylcholine in the olfactory bulb when HDB input to the olfactory bulb is activated electrically or chemically,
21 which is thought to affect the excitability of granule and periglomerular cells.
22 Cholinergic input to the piriform cortex is thought to increase the excitability of neurons and the plasticity of synapses in this region. Conversely, changes in the activity of piriform cortex neurons associated with odor familiarity/novelty have been shown, computationally and experimentally, to modulate incoming cholinergic input through feedback mechanisms.
18 Interactions within this complex system of cholinergic circuits may be relevant to our findings, since olanzapine is known to produce increases in cholinergic function via antagonism of M2 autoreceptors. In a recent study, 12-week exposure to the acetylcholinesterase inhibitor donepezil improved accuracy on the 40-item UPSIT [the University of Pennsylvania Smell Identification Test] in elderly individuals.
23In addition to direct modulation of olfactory function, olanzapine’s mood-stabilizing, and potentially anxiolytic,
24 properties may be relevant to our clinical observations by contributing broadly to a reduction in parosmia and an elevation in mood. Previous work has shown that patients with parosmia for more than 2 years have smaller olfactory bulbs and marked gray matter loss in the left anterior insula, right anterior insula, anterior cingulate cortex, bilateral hippocampus, and left medial orbitofrontal cortex,
25 suggesting that parosmia may be an emergent symptom of interpreted neural circuit dysfunction across multiple brain regions in the limbic system. The size and location of the olfactory tubercle in human brain preclude a detailed analysis of this region via MRI
26 in patients with parosmia.
Further studies (e.g., using odor-evoked functional MRI) conducted in ORS patients may reveal the mechanisms with which olanzapine affects parosmia and olfactory processing. Moreover, a longer drug exposure may produce longer-lasting effects and a more profound reduction of parosmia throughout the olfactory battery. Taken together, our findings indicate that olfactory function is compromised in our ORS patient, the experience of parosmia worsens odor identification, and olanzapine therapy may offer olfactory functional benefits in conjunction with symptomatic relief of parosmia in patients diagnosed with ORS.