Death by suicide is a tragic event that occurs at higher rates among individuals with a psychiatric disorder, particularly mood disorders.
1 However, it remains unclear why not all depressed patients who have suicidal ideation attempt suicide. Theoretical frameworks of suicidal behavior, such as the stress-vulnerability model, indicate that suicidal behavior may result from an interaction between exposure to stressors and a vulnerability in individuals suffering from psychiatric disorders.
2 Suicide attempts have been associated with anhedonia and deficits in reward processing in both elderly
3 and adolescent
4 attempters when compared with nonattempters.
Studies on the neurobiology of suicide suggest that interrelated neurobiological systems linked to dysfunction in the stress response systems are involved. The corticotropin-releasing hormone/hypothalamic-pituitary-adrenal axis and the locus coeruleus-based norepinephrine system, which interact with the serotonin and opioid systems, are likely to be involved.
5 Although they are usually small and heterogeneous, neuroimaging findings report associations between suicidal ideation as well as suicide-related behaviors with abnormalities involving the frontal neural system and the serotonergic system, including the orbitofrontal cortex, the anterior cingulate cortex, and the amygdala.
6–9 Taken together, these findings suggest that brain regions regulating the stress response, as well as the reward-dopaminergic and serotonergic systems, may be involved in the circuitry underlying suicidal ideation and suicide-related behaviors.
One likely locus of concerted control over multiple neurobiological systems is the habenula, a region that links the forebrain to the midbrain. Indeed, because of its unique anatomical and functional position, the habenula modulates downstream limbic midbrain dopamine and serotonin circuits by controlling activity in the monoaminergic nuclei, the substantia nigra compacta/ventral tegmental area, and the raphe nucleus.
10 Thus, as a relay interface between the basal ganglia and the limbic system, the habenula is involved in both motivational and emotional control of behavior,
11 playing a critical role in both the stress response and the behavioral responses induced by expected reward.
12,13 Not surprisingly, increasing evidence suggests the involvement of the habenula in the pathophysiology of a variety of psychiatric disorders, including mood disorders, schizophrenia, and substance use disorder.
14,15 Specifically, increased connectivity and activation of the habenula has been associated with depression
16,17 as well as sleep disturbances.
18 It is not known, however, whether the habenula is involved in suicidal ideation and suicide-related behaviors.
The aim of the current study was to compare habenula resting state functional connectivity (rsFC) in a sample of patients with mood disorder, with and without a history of suicide-related behavior and healthy control subjects. We hypothesized that higher connectivity of the habenula with limbic regions would be associated with suicidal ideation and suicide-related behaviors.
Methods
Participants
The Baylor College of Medicine Internal Review Board approved the study protocol and procedures. All participants provided informed consent.
Inpatients who met criteria for current major depressive episode and with a primary DSM-IV-TR diagnosis of either major depressive disorder, recurrent (MDD; N=165) or bipolar disorder type I or type II (N=32) were recruited for the study. They were culled from a sample of 316 inpatients who gave their consent to take part in the study after being admitted at The Menninger Clinic in Houston. Participants with comorbid substance use disorder diagnoses were included, given the pervasive comorbidity between mood and substance use disorders
19 and the need to investigate ecologically valid samples in neuroimaging studies.
20 Psychiatric diagnoses were made using the Structured Clinical Interview for DSM-IV Disorders.
21,22 Tobacco, alcohol, and cannabis were the most prevalent substances of abuse in the sample, according to the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test. All patients were medicated at the time of the scanning.
Healthy control (HC) subjects were recruited from the general population to serve as comparators for the rsFC analyses. HCs were excluded if they had a self-reported history of psychiatric diagnosis or substance abuse. Additional exclusion criteria for both patients and controls were a previous history of traumatic brain injury with loss of consciousness of more than 10 minutes or any other MRI contraindication.
Clinical Measures
Participants completed a comprehensive diagnostic assessment. Suicidal ideation and suicide-related behaviors were assessed using the Columbia Scale for Rating of Suicide Severity (C-SSRS).
23,24 The C-SSRS is a semistructured interview that assesses severity of suicidal ideation, intensity of ideation, suicidal behavior and associated lethality of suicide attempts. The Suicidal Ideation (SI) subscale consists of five items: a passive wish to be dead; nonspecific active thoughts of suicide; active suicidal ideation, including methods; suicidal intent without a specific plan; and active suicidal ideation with intent and a plan to act. The Intensity of Ideation subscale rates the frequency, duration, controllability, deterrents, and reasons for thoughts of suicide. The Suicidal Behavior (SB) scale assesses past actual attempts, past nonsuicidal self-injurious behavior, past interrupted attempts, and past aborted attempts. The lifetime SB subscale was used to stratify groups. Patients were categorized in the suicide-related behaviors group (SB+) if they responded affirmatively to any one of the following three items: actual attempt, interrupted attempt, or aborted attempt. They did not meet criteria for inclusion in the SB+ group if they responded affirmatively solely to the following items: self-injurious behavior and preparatory acts or behavior. This approach is consistent with previous studies.
25,26 Patients were included in the non-suicide-related behaviors group (SB–) if they had positive response to any item of the SI but negative response to all items of the SB. The final sample groups included 123 patients categorized as SB+ and 74 as SB–.
Depression severity was assessed with the self-rated nine-item Patient Health Questionnaire (PHQ-9) depressive scale.
27 The PHQ-9 is a reliable measure of depression severity that scores each of the nine diagnostic criteria of major depressive episodes over the past 2 weeks as “0” (not at all) to “3” (nearly every day).
28Neuroimaging Data Acquisition and Preprocessing
Participants underwent magnetic resonance imaging of the brain on a 3T Siemens Trio Magnetom system. A structural T1-MPRAGE (TR=1,200 ms, TE=2.66 ms, flip angle=12°, voxel size=1mm isotropic, field of view=245 mm, and total sequence time=4.5 minutes) was first acquired. A 5-minute resting-state echo-planar imaging scan (echo time=30 ms, repetition time=2,000 ms, flip angle=90°, voxel size=3.4×3.4×4 mm, field of view=220 mm) was subsequently acquired for each subject. During the resting-state sequences, a large X was displayed on the screen; participants were asked to keep their eyes open or closed and not fall asleep.
Preprocessing of the functional data, including functional realignment and unwarp, functional slice-timing correction, structural segmentation and normalization, functional normalization, ART-based functional outlier detection and scrubbing, and functional smoothing (8-mm Gaussian kernel), were done in Montreal Neurological Institute (MNI)-space using CONN-fMRI Functional Connectivity toolbox v15.b
29 with SPM8 (
www.fil.ion.ucl.ac.uk/spm/). The Artifact Detection Toolbox (ART;
http://www.nitrc.org/projects/artifact_detect/) was used for outlier detection and scrubbing as implemented in CONN. Motion outliers were defined as any frame where the motion exceeded 2 mm. Realignment parameters were entered as first-level covariates in the toolbox. Realignment, scrubbing, white matter, and cerebrospinal fluid were entered as potential confounders in the subject-level general linear model. Using the anatomical component correction (aCompCor) method of flexibly removing physiological noise and movement confounds on a voxel-by-voxel level,
30,31 each of these effects was regressed out of the blood-oxygen-level-dependent (BOLD) signal before connectivity measures were computed by the toolbox. Functional images were then temporally band-pass filtered (0.01<
f <0.1 Hz) to investigate low frequency correlations. Since we were interested in the habenula, which is a very small region, we downsampled the images to 3×3×3 mm. In addition, the habenular seed in rsFC analysis was not smoothed, to avoid likely contamination with adjacent areas.
Functional Connectivity Analyses
We first examined the rsFC between each habenula region of interest and each voxel of the brain. The left and right habenula seed regions of interest were manually created for every subject by using the T
1 image to visually identify the right and left habenula of each subject’s landmarks with SPM8 (
www.fil.ion.ucl.ac.uk/spm/). In T
1 images, the habenula is clearly visible as two small triangular structures pointing into the third ventricle. Each functional region of interest was a 3×3×3-mm cube placed around a central MNI coordinate in the habenula.
The mean signal time course from the seeds was extracted and the Pearson’s correlation coefficients with the time course of all other voxels of the brain were calculated. Correlation maps were calculated for each subject and correlation coefficients were then converted to normally distributed z-scores using the Fisher’s transformation in order to perform general linear model analyses. In order to compare rsFC across the three groups (SB+, SB–, HC), assuming as the null hypothesis that the three groups would show equal rsFC with the left and right habenula seeds, analysis of covariance (ANCOVA) was performed with age and gender included as covariates. A voxel statistical height threshold of p<0.001 with a cluster threshold of p<0.05 family-wise error corrected was used to identify connectivity differences between the three groups. Significant clusters identified in this analysis were then imported as target regions of interest in the post hoc region of interest to region of interest analysis. The connectivity values between the significant clusters and left and right habenula were extracted from each participant in order to examine the nature of the connectivity in each group and the directionality of each post hoc contrast. The results were considered significant if p<0.05, false discovery rate corrected. To further explore whether additional factors such as MDD/bipolar disorder diagnosis, substance use/abuse disorder, or medications might have affected the significant results in the between patient group comparison (SB+ compared with SB–), we subsequently included these covariates in the model in a post hoc manner to avoid reducing statistical power of the original ANCOVA. Thus, mood disorder diagnosis, alcohol and substance use/abuse disorder, and the effects of antidepressants, lithium, antiepileptics, anxiolytics, and antipsychotics were entered as dichotomous covariates (absent versus present).
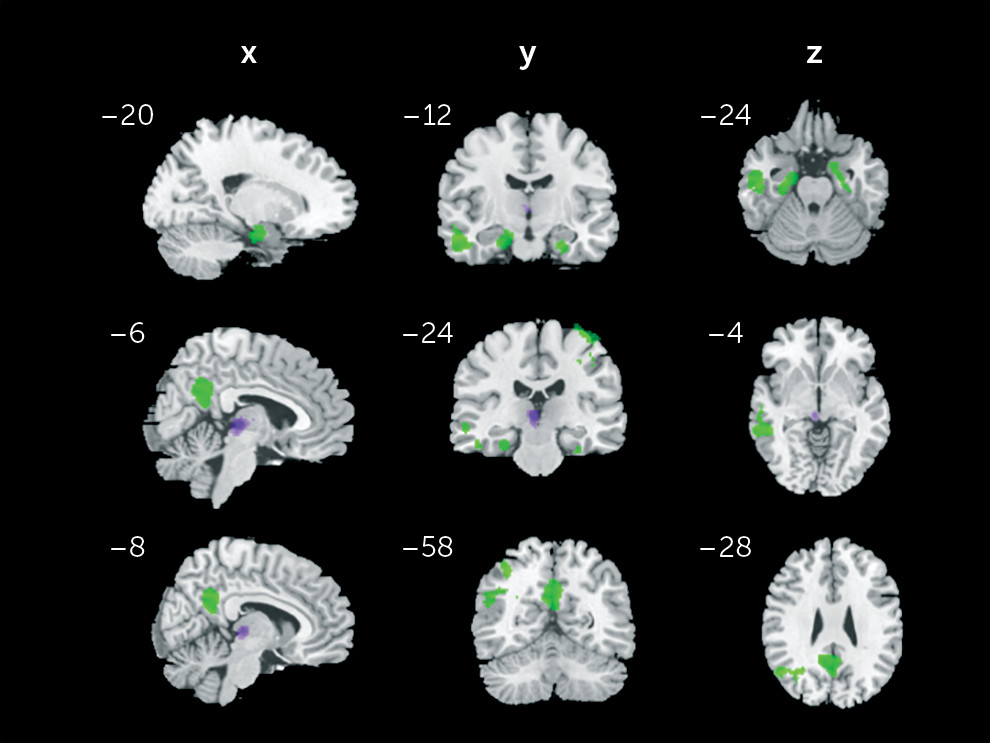
Additionally, to address whether the significant findings (clusters displayed in
Table 1 would be specific to the habenula signal, we conducted an exploratory SB+ compared with SB– region of interest to region of interest comparison including thalamic regions as seeds. Specifically, the whole bilateral thalamus based on the automated anatomical labeling atlas
32 as well as two small bilateral thalamic control regions of interest were chosen as seeds. The two small bilateral thalamic seeds were created by shifting the averaged habenula region of interest coordinates by 9 mm in the anterior direction (“thalamus medial,” MNI: right 4, –15, 2; left –3, –16, 2), and in the lateral direction (“thalamus lateral,” MNI: right 13, –24, 2; left –12, –25, 2). The results were considered significant if the p value was <0.05 (false discovery rate corrected).
Discussion
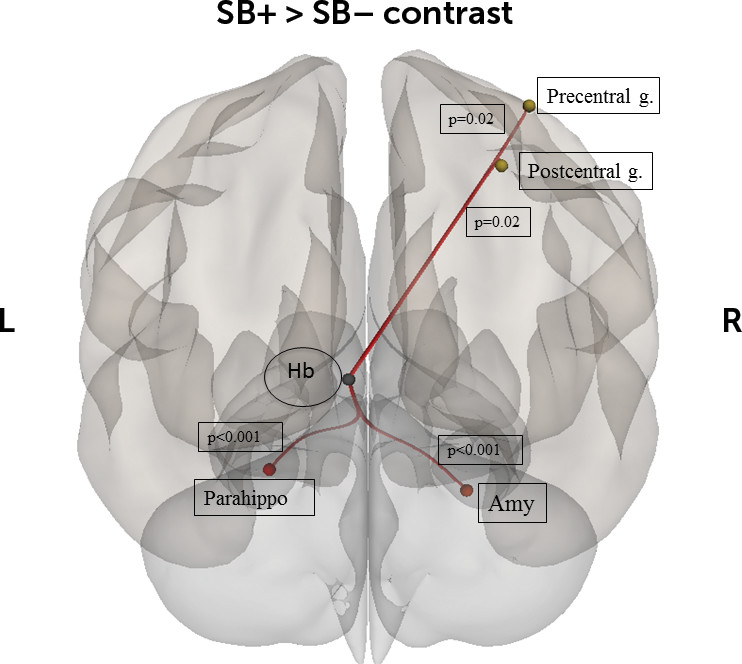
The present study investigated habenular rsFC differences between patients with a diagnosis of mood disorder, with and without a history of suicide related-behaviors, and healthy controls. To the best of our knowledge, it represents the largest study investigating neuroimaging correlates of suicidal-related behaviors in a population of mood disorder patients. As hypothesized, we found that the SB+ group had higher connectivity between the left habenula and several regions: left habenula and left parahippocampal gyrus, left and right amygdala, and right precentral and postcentral gyri. Moreover, patients with a diagnosis of mood disorder, independently of suicide-related status, displayed higher connectivity between the left habenula and middle temporal gyrus, angular gyrus, and PCC as well as decreased connectivity between the right habenula and left thalamus. The present results suggest that altered connectivity of the habenula with regions of the limbic and motor systems might be involved in the neural basis of suicide-related behaviors.
Typically described as composed by medial and lateral subregions, the habenula controls behavior, cognition, and emotion through extensive inputs from the basal ganglia and the limbic regions.
33 Based on these inputs, the fasciculus retroflexus carries outputs that target dopaminergic and serotonergic neurons.
12 By inhibiting the firing activity of the dopamine and serotonin systems, the habenula facilitates the evolutionary mechanism of action avoidance when the outcome is less rewarding than predicted.
11Our data support the evidence that altered activity of the habenula contributes to the etiology of psychiatric disorder, including depression.
34 Evidence that the habenula is hyperactive during depression comes from animal models of depression
33 and pharmacological studies.
35 Neuroimaging studies of habenula activity in patients with MDD showed contrasting results. Although increased activity of the habenula was detected in remitted patients with MDD,
36 reduced activity was observed in depressed MDD during the prediction of negative outcome
37 and primary aversive conditioning.
38 The investigation of an association between habenula connectivity and suicide is new to the literature. However, the higher connectivity of the left habenula we found in the SB+ group is somewhat in line with the disrupted habenula function observed in depression. Thus, it is tempting to speculate that in the SB+ group the higher connectivity with regions of the limbic and motor systems might be related, at least partially, to a possible increased sensitivity of habenula cells induced by chronic stress.
12,39,40 Although the exact mechanism that links suicide and chronic stress is not fully understood, stress-related alterations in cell structure have been documented in suicide attempters.
41 Chronic dysregulation of the habenula circuit may lead to long-term alterations of dopamine, serotonin, and norepinephrine release that impact behavioral functioning, particularly the learning of maladaptive coping strategies related to suicide-related behaviors.
42 Interestingly, the parahippocampal gyrus has been linked to suicidality: in an fMRI experiment using a delay discount task, suicidal patients showed longer reward delays were associated with diminished parahippocampal response.
43 The parahippocampal cortex has been hypothesized to be a critical part of a network of brain regions that processes contextual associations.
44 This makes functional connectivity with the habenula a possibly important feature of contextual associations in general, and especially those that include reward as an important feature.
Interestingly, the left habenula was strongly associated with suicidality, while the right habenula was much less involved. Habenular asymmetry has been shown to be critically important for behavior in fish,
45 and more recently, rsFC of the habenula in healthy humans was shown to be asymmetric.
46 Our data suggest that habenular asymmetry may also be important for psychiatric disorder etiology, which may have implications in terms of future therapeutic intervention development, especially for neuromodulatory approaches. For example, deep brain stimulation of the habenula has been shown to be possibly therapeutic in treatment-resistant MDD.
47 This might imply that unilateral habenula stimulation, which arguably could have lesser side effects, should be explored.
Not surprisingly, significant results were also found when the medial thalamic seed was considered. While we cannot exclude this might be partially attributable to the spatial distortion of the MRI images, it could also be attributable to shared connectivity patterns between the habenula and the medial nuclei of the thalamus. Overlapping and mutually interacting circuits (e.g., similar inputs from the ventral/limbic striatum) between the two regions have already been described.
17,48It is important to address several limitations in the present study. First, the reliability of in vivo study of the habenula through fMRI has been debated,
17,49 considering the small size of this structure. We tried to temper this caveat using individual-specific habenula regions of interest, without attempting to isolate the medial and lateral subdivisions. However, we cannot exclude that head movement as well as spatial resolution and smoothing might have contributed to a signal contamination from the surrounding regions. Second, the current study examined suicide behavior in a mixed sample of depressed patients diagnosed with MDD or bipolar disorder. Although this study includes patients with diagnostic heterogeneity, the two disorders tend to be more similar than not when it comes to suicide-related behaviors.
50 All individuals in the SB+ and SB– groups were prescribed multiple medications at the time of MRI. Although medication status was secondarily included as covariates in the analyses performed here, the potential effects of medication status on rsFC cannot be disentangled fully from those effects attributable to suicidal behavior. It is noteworthy that in a recent review of the literature,
51 the authors concluded that medication effects do not seem to provide an alternative explanation for observed differences in BOLD signal.
In summary, the present findings suggest that the habenula might be involved in the neural circuitry of suicide. As such, the higher habenular rsFC we found in the suicide-related behaviors group may mediate a dysfunction in the critical survival mechanism by which the habenula works as a suppressor of motor activity. Nonetheless, additional research focused on the identification of neuroimaging differences between patients with only suicidal ideation from those with suicide-related behaviors is needed in order to identify patients at higher risk to attempt suicide.