Delirium is an acute impairment of consciousness that presents as a stereotyped response of the brain to a diverse range of medical, surgical, and pharmacological conditions.
1 It is characterized by impaired consciousness presenting clinically as fluctuating alteration of higher-cortical functions with prominent inattention, and its diagnosis excludes states such as stupor and coma, in which consciousness is lost. Empirical research has shown that there are three core characteristic symptom domains comprising delirium, while some other symptoms are less consistent and therefore less defining. These three symptom domains are cognitive (orientation, attention, short- and long-term memory, and visuospatial ability), higher-order thinking (language, thought process, and executive function), and circadian (sleep-wake cycle and motor activity alterations).
2–5 Delirium has three motor presentations (hyperactive, hypoactive, and mixed) that appear to remain consistent throughout an episode in more than one-half of patients.
6 Affective lability and psychotic features are noncore symptoms and considered accessory because they occur less frequently and are nonspecific.
5 Features of delirium that differentiate the syndrome from other conditions are acute temporal onset, fluctuation of symptom severity, and attributable physical etiologies.
2–6How delirium presentation may be affected by developmental stage is not well studied. Most research on delirium phenomenology has been conducted with adults, and much less is known about pediatric patients.
7 Geriatric patients may have comorbid degenerative or vascular cognitive disorders, which are risk factors for delirium. Most research shows that when they are comorbid with preexisting cognitive disorders, delirium features predominate, but with greater overall burden of cognitive impairment.
8–14The brain undergoes changes during normal aging, especially notable from the seventh decade of life, and those changes can cause reduced cognitive reserve. At a macroscopic level, there is brain weight loss up to 200 g, accompanied by gyri narrowing, sulci widening, and dilatation of ventricles. From a microscopic perspective, there is some neuronal loss. Neurofibrillary tangles, diffuse plaques, and neuritic amyloid plaques can appear. Marinesco (substantia nigra) and Hirano (especially at hippocampus) bodies and diverse vascular changes may be present.
15Turkel et al. reported that sleep-wake disturbance, fluctuating symptoms, impaired attention, irritability, agitation, affective lability, and confusion were more often noted among children; impaired memory, depressed mood, speech disturbance, delusions, and paranoia were relatively more common among adults; and impaired alertness, apathy, anxiety, disorientation, and hallucinations occurred similarly in both populations.
16 Leentjens et al. reported that delirium among adult (mean age, 55.4 years) and geriatric (mean age, 76.2 years) patients had similar features when assessed with the Delirium Rating Scale (DRS), except that geriatric patients had more severe cognitive impairment.
17 Grover et al. found that the prevalence and severity of symptoms of delirium, assessed with the DRS–Revised–98 (DRS-R-98), were similar across adult and geriatric groups (cutoff=65 years), except the adult group had higher prevalence and severity for thought process abnormalities and lability of affect. For both age groups, factor analysis revealed a three-factor model where loading for the 16 DRS-R-98 items showed only subtle differences across factors.
18 Although there are few comparative reports, there is a suggestion that age and, inferentially, the stage of brain development, aging, and degeneration may affect the pattern of delirium symptom severities across different age groups.
Whether sex plays a role as a determinant of delirium clinical presentation is also not understood. Biological sex influences may affect clinical characteristics of some neuropsychiatric disorders, such as schizophrenia,
19 bipolar disorder,
20 or Alzheimer’s dementia.
21 In general, under normal conditions, women tend to have better verbal abilities, whereas men have better visuospatial ability.
22–24 In Alzheimer’s dementia, women have more impaired visuospatial ability and worse verbal memory than men, despite their sex-specific greater cognitive reserve on this domain.
21,25 Possible explanations are that the negative impact of apolipoprotein E ε4 allele is greater among women with Alzheimer’s disease than among men
26 and that estrogen deficiency among older women with Alzheimer´s disease is associated with worse verbal task performance.
27 Although point prevalence is quite similar for male and female delirium cases (50.9% for male),
28 to our knowledge there are no studies evaluating sex differences for characteristics of delirium.
The aim of this study is to describe the delirium phenotype according to nongeriatric and geriatric age groups and sex in a large data set of adults without dementia or major psychiatric diagnoses.
Methods
Data Set
This pooled data set was composed of deidentified individual data of adult patients with delirium, who were without dementia or other major psychiatric comorbidities. Data were pooled from primary studies in seven countries that utilized comparable patient assessments.
Patients with delirium in the primary studies were identified through different referral approaches, although all were diagnosed by delirium experts using DSM-IV criteria and nearly all were hospitalized in general hospital settings. They were recruited as follows: Trzepacz et al.
29 blindly assessed neuropsychiatric patients from several inpatient clinical settings for delirium in a quasi-randomized way; Lim et al.,
35 Franco et al.,
30 and Kean et al.
38 drew participants from consecutive admissions to general hospital or rehabilitation hospital units; Meagher et al.
36 and Kato et al.
33 worked with consecutive general hospital patients referred for psychiatric assessment by the treating team; participants in the De Negreiros et al.
31 study were general hospital patients with suspected mental status change referred by their medical team; Lee et al.
37 worked with consecutive general hospital patients referred to the psychiatry liaison service; Huang et al.
32 and Lee et al.
34 assessed general hospital patients with delirium recruited by the liaison psychiatry unit; and participants in the Leonard et al.
3 study were consecutive general hospital patients screened for delirium.
The authors were invited to participate by the developer of the DRS-R-98 (Dr. Trzepacz)
29 because they had permission to perform further validation studies of the scale, and their work used the same criteria. All sites’ researchers used delirium experts who were well trained and experienced in using the DRS-R-98. Delirium and other neuropsychiatric diagnoses, including dementia, were made according to DSM-IV or DSM-IV-TR and drew on all available sources of clinical information (no other specific diagnostic tool was applied for detecting dementia). The researchers performed DRS-R-98 ratings blind to DSM diagnoses. All patients were evaluated cross-sectionally with both DSM and DRS-R-98. All studies had to be approved by the appropriate human ethics committee at their sites, and informed or proxy consent was obtained as required.
Participants
A total of 406 patients with delirium (United States N=45, Brazil N=19, Colombia N=8, Japan N=29, Taiwan N=8, Korea N=193, Ireland N=104) were assessed from a variety of inpatient and outpatient facilities. Age and sex data were collected. Active medical diagnoses from the original databases were coded in a standardized manner according to the categories of the Delirium Etiology Checklist, but without attribution to delirium causality per se.
39Measures
Delirium characteristics were measured with the DRS-R-98. The scale was developed on the basis of the well-established characteristics of delirium.
29 Although it is not derived from a specific diagnostic system, its items and diagnostic ability accord well with the diagnosis of delirium independently of DSM-III-R, DSM-IV, DSM-IV-TR, DSM-5, and ICD-10 criteria use and may be utilized to provide delirium diagnosis accordingly.
40The DRS-R-98 is a 16-item observer-rated diagnostic and severity rating scale with phenomenologically descriptive anchors for each severity rating level of each item. Increasing item scores correspond to more severe symptoms (0=normal, 1=mild, 2=moderate, 3=severe). Of the 16 items in the total scale, 13 comprise the severity scale, and three items assess diagnostic characteristics. Symptoms evaluated by the severity scale include 10 items representing the three core domains of delirium (cognitive, higher-order thinking, and circadian) and three items representing the noncore or accessory symptoms (psychotic symptoms and affective lability). The DRS-R-98 diagnostic characteristics comprise three items: temporal onset, fluctuation, and attribution to possible etiologies.
29The maximum score of the DRS-R-98 total scale is 46 points. The DRS-R-98 is not derived from any diagnostic system and was developed on the basis of known characteristics of delirium. Additionally, for some analyses in this report, subscores representing the cognitive domain (orientation, attention, short-term memory, long-term memory, visuospatial ability), higher order-thinking domain (language, thought process), circadian domain (sleep-wake cycle, motor agitation, and motor retardation), and psychotic symptoms (perception and delusions) were collapsed into variables whose subscores represented them (see the Statistics section below). Motor items (agitation and retardation) were used to define the motor presentation of delirium; patients with item scores ≥1 were considered either hyperactive or hypoactive, respectively, or mixed (when they scored positive on both items).
The DRS-R-98 original version was validated by raters blind to diagnostic status. It was revalidated as translated versions in many languages and countries. The data for this analysis were obtained with the version of the scale corresponding to the language of each study country. All the versions of the scale used here have excellent interrater reliability and accuracy (intraclass correlation index and area under the ROC curve >0.9). Information about translations and validations is reported in the DRS-R-98 administration guide (pdf available from Dr. Trzepacz,
[email protected]).
39Statistics
General bivariate analyses.
The bivariate analyses were performed according to dichotomous age groups (<65 or ≥65 years) and, separately, by sex groups (male and female). Mean age and mean DRS-R-98 scale and domain scores were compared with t tests. DRS-R-98 individual items were compared with the median test. Sex, medical diagnoses, and frequency of motor types of delirium were compared with chi-square tests.
Multivariate analyses.
We performed multivariate analyses of DRS-R-98 scores using exploratory factor analysis to delineate the delirium construct for age and sex groups. Factor analysis is a multivariate method of modeling the relationship among observed characteristics (variables) that produces a smaller number of latent variables called factors. The factors represent clusters of the observed variables that correlate (load) highly with each other and enable researchers to discern underlying constructs. The factors typically are viewed as broad concepts or ideas that may describe an observed phenomenon.
We performed factor analyses as our multivariate exploration of correlations among delirium characteristics within each of the two dichotomized groups—by sex and by age. Furthermore, we performed two different sets of factor analysis for each of these four demographic groups. The first factor analysis included only DRS-R-98 subscores, whereas the second factor analysis included DRS-R-98 subscores with the addition of control variables, sex (when we analyzed the two age groups), age (when we analyzed the two sex groups), principal medical diagnoses whose frequency was ≥10% in that particular analysis group, and presence of one or more additional medical diagnoses in each one of the four groups. To reduce variability, we factor analyzed delirium characteristics by using DRS-R-98 subscores for each of the three core domains (cognitive, higher-order thinking, circadian) and scores for each of the accessory symptoms (psychotic items and affective lability) and diagnostic items (temporal onset, fluctuation, and attribution to possible etiologies), which provided eight variables instead of 16.
The determinant of the correlation matrix was used to determine whether the variables were appropriate for factor analysis in this sample (expected value near 0). Bartlett’s test of sphericity (χ
2<0.05) and Kaiser Meyer Olkin measurement (>0.5 according to Kaiser criterion) were used to determinate whether principal-components analysis was appropriate. We selected factors by assessing the inflection point of the scree plots of eigenvalues. For both sets of analyses, Varimax rotation was made for distributing variance and reducing factorial complexity without producing interaction among the selected factors. Finally, to establish which variables significative loaded in each factor, the formula
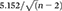
was used (5.152 is the double of 1% level of significance in the normal curve, and n is the number of subjects for each study category).
Results
Mean age for the full sample of 406 delirium patients was 68.0±14.96 (range=18–100), and 258 (63.5%) were male. Other characteristics of the pooled data set are shown in
Table 1. As expected, when the sample was divided according to an age cutoff of 65 years, there was a significant difference for mean age, with 25.63 years difference between geriatric and adult groups. All other characteristics compared by age were similar, except for two principal admission diagnoses: systemic infection (more frequent in the geriatric group), and traumatic brain injury (TBI; more frequent among nongeriatric adults). Hypoactive delirium was more frequent among women.
Table 2 shows that there were no significant differences for delirium domains or DRS-R-98 severity and total scale mean scores between age groups. The diagnostic items each had significant slightly higher mean scores for nongeriatric adults versus geriatric adults and for males versus females. However, there were a few significant differences by sex. Males had a higher score on the circadian domain and accessory symptoms than females and on the DRS-R-98 severity and total scale mean scores.
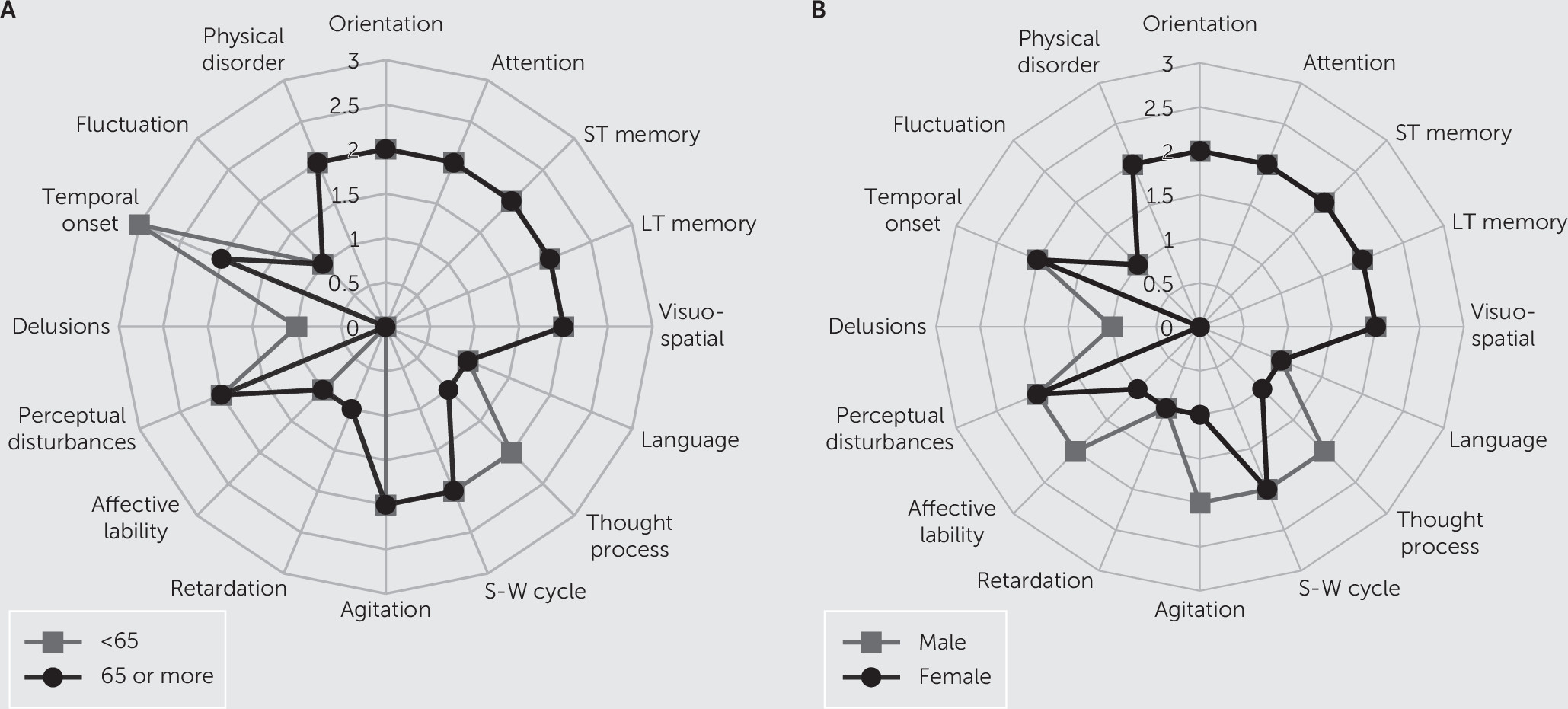
According to
Figure 1 radar graphs, symptom patterns looked very similar between the age and sex groups, with a few differences. The median scores for motor agitation and affective lability items were each higher by one severity point among males than females. Regarding diagnostic items, temporal onset was more acute among nongeriatric adults than geriatric adults, and geriatric adults also scored higher on the delusion item. Although the thought process item looked different between age groups, it was not significant.
Factor Analyses
The factor analysis scree plots’ inflection points occurred in the second component for almost all groups analyzed, except for when control variables were included when the inflection point occurred in the third or between the second and the third in the nongeriatric adult, male, and female groups (see Figure S1 in the
online supplement). The third component for these three groups explained less than 10% of the variance. Therefore, we decided to choose a parsimonious solution for all groups that contained two factors (named F1 and F2). All eigenvalues of included factors were >1.
Table 3 shows rotated factor analyses of all delirium characteristics, which also appear in
Table 4 with control variables.
Usually the core domains loaded onto F1, except for males and the geriatric group when control variables were not considered in the factor analysis. Accessory symptoms were loaded onto F1, but in the demographic groups, when the circadian domain loaded onto both F1 and F2 (male and geriatric groups), then the accessory symptom items also loaded onto F2. Regarding female and nongeriatric groups, the relationship of core domains and accessory symptoms were quite similar, regardless of whether control variables were considered in the factor analysis.
The influence of the active medical diagnoses on factor loadings was complex, with many negative loading values and small loadings that were not significant in many cases. Almost all loadings were only onto F2 (the factor with the lower percentage of variance explanation). However, there was a clear influence of a few principal diagnoses on factor loadings. TBI loaded together with the cognitive domain and temporal onset item onto F2 for nongeriatric adults, whereas metabolic and endocrine disturbances loaded onto F2 in all the other demographic groups. Male and geriatric groups had more active medical diagnoses that loaded onto their factors, including having multiple medical diagnoses (i.e., the presence of at least one other active diagnosis), consistent with a higher medical morbidity burden.
Finally, when age was controlled together with active medical diagnoses in the sex groups, it loaded onto F2 in both groups. Conversely, sex did not load onto any factor in geriatric or nongeriatric adult groups.
Discussion
We performed analyses of the impact of adult age groups and sex on delirium phenotype using the DRS-R-98 items, including factor analysis when items were subgrouped according to the three core domains, accessory symptoms (psychosis and affective lability), and diagnostic items. Our data were pooled from international research sites, and only nondemented delirious patients without current major psychiatric diagnoses were included. Overall, we found few significant differences between groups and confirmed a two-factor structure for delirium phenomenology.
There were only a few significant differences between age or sex groups in the mean and median DRS-R-98 scale and item comparisons. Most notable was the behavioral presentation in males having higher total and severity DRS-R-98 scores with greater motor agitation and affective lability scores than females, who had a higher prevalence of hypoactive delirium. Nonetheless, the three core domains were otherwise comparable, except for the circadian domain, which was more severe in the male group, attributable to the higher score for motor agitation. Diagnostic characteristics scores were only slightly, although statistically, higher in male and nongeriatric adult groups versus their demographic dichotomized counterparts.
Our two-factor solutions resulted for all four groups, dichotomized by the demographic variables of age and sex. This two-factor solution is consistent with previously reported exploratory factor analyses in delirium that did not examine groups according to demographic variables.
2,41 It is also consistent with the two factors in a confirmatory factor analysis of DRS-R-98 items.
5 However, there have been two DRS-R-98 factor analysis studies that reported three-factor solutions.
42,43Additionally, all previous factor analyses of DRS-R-98 items support the construct of three core domains and noncore (accessory) characteristics, as utilized by us for this study. All three core domains in our report loaded onto either of the two factors in all four demographic groups, and accessory symptoms loaded together with the core domains for younger adults and females. However, in the geriatric and male groups, the accessory symptoms loaded onto the same factor only with the circadian core domain, whereas the cognitive and higher-order thinking domains loaded together on the other factor (see
Table 3). This suggests that in these four demographic groups, the three core domains do not all load on the same factor together for some reason, perhaps related to underlying brain function circuitry or prevalence of certain delirium etiologies that may be driving accessory symptoms to align only with sleep and motor presentation. Alternatively, the presence of these accessory symptoms might secondarily affect behavior to disturb the normalcy of motor activity periods and sleep-wake cycle continuity in certain demographic subgroups.
Delirium was described by Bonhoeffer at the beginning of the 20th century as a stereotyped and generalized reaction of the brain to exogenous causes, regardless of etiology.
44 However, some medical conditions can have notable influence on phenomenology–this is known for alcohol withdrawal delirium, where motor hyperactivity and hallucinations are more common than in other delirium etiologies.
45 Cognitive alterations (attention and memory alterations) and acuity of onset are very prominent in TBI delirium, which may also often include motor agitation and hallucinations.
38 Similarly, we found that when we controlled for other medical factors and for sex (see
Table 4), TBI loaded among nongeriatric adults onto the same factor with the cognitive domain and with temporal onset. In the geriatric group, systemic neoplasm loaded onto the same factor as accessory symptoms. Among males and females, metabolic and endocrine diagnoses had high loadings onto F2, but it is difficult to make much sense out of what that might mean for delirium phenomenology. Most diagnoses had low loading values, and a few were inverse. In the overall delirium literature there is a paucity of careful study of how particular etiologies influence delirium presentation other than the common, somewhat stereotyped observation for ethanol withdrawal delirium.
Regarding influence of the two demographic variables over each other, we found a one-way relationship where age behaved in an independent manner in both sex groups where it was correlated with F2 when control variables were considered in the factor analysis. However, this correlation was low and complicated, because those two F2s explained the least amount of variance of all factors reported, age loaded inversely with F2, and other control variables also loaded together with age. This may be related to the impact of aging in the brain and hormones, where cognitive reserve declines and neuroprotective effects of testosterone, progesterone, and estrogen are lost over the years among males and females.
46This study has limitations that should be highlighted. Delirium cases in our pooled database were recruited through both referral and consecutive case identification approaches, although mostly from general hospital settings, which might have contributed some bias or heterogeneity in some difficult-to-quantify way. Conversely, all included delirium cases were diagnosed according to DSM-IV and DSM-IV-TR criteria by delirium experts. We report a cross-sectional assessment of patients and so cannot describe evolution of delirium characteristics over the course of episodes. However, our patients had an ample spectrum of delirium severity, and longitudinal studies report that delirium symptoms are consistent during an episode.
6 We did not perform a separate evaluation of attributable delirium etiologies; rather, we utilized the main active medical diagnoses as control variables, so we cannot affirm that there was a definitive causal relationship between those diagnoses and delirium. Nevertheless, it is quite possible that those active diagnoses led to hospitalization and were contributors to the patients’ delirium.
To the best of our knowledge, this is the first report of both age and sex demographic variables that considers the effect of each one over the other and the effect of other clinical variables, such as active medical diagnoses. Future studies that specify a priori demographic and delirium etiology groups are needed to replicate or refute our findings.
In conclusion, exploratory factor analysis of the three-core-domain model of delirium with psychotic and affective accessory symptoms yielded a two-factor construct for delirium phenomenology that was independent of sex or age when dichotomized at 65 years. We found only a few phenomenological differences in factor loadings, which suggests that delirium presentation is largely comparable across age and sex. Males tended to have higher scores on motor agitation and affective lability, and the circadian core domain (motor and sleep-wake cycle alterations) covaried with accessory psychotic and affective symptoms. The influence of the active medical diagnoses was complex, but with a clear influence of TBI with cognitive alterations and abrupt onset of delirium. Age was mildly related to delirium characteristics in both male and female groups.