Tremor is defined as “an involuntary, rhythmic, oscillatory movement of a body part.”
7 One useful approach to classification describes four main features: anatomical region affected (e.g., the mouth, leg, trunk, or hand), activation (resting, intention, postural), frequency (how fast the motion, typically described in Hz), and amplitude (how large the motion).
12 Tremor can also be defined by etiology (i.e., as part of a disease symptom cluster), such as an exaggerated physiological tremor, an essential tremor (ET), Parkinson’s disease, Wilson’s disease, a drug-induced syndrome, or a psychogenic illness. ET is considered the most common movement disorder.
13,14 Although classically conceptualized as a homogeneous “benign” motor disorder, an increasing body of research indicates that ET is neither a single entity nor benign. The most recent consensus guidelines consider ET to be a family of disorders and differentiate ET from ET plus.
7 ET is characterized by the presence of bilateral upper-extremity action tremor (postural and/or kinetic) with or without tremor in other locations (e.g., the head, voice, or lower limbs) of at least 3 years duration with absence of other neurological signs.
7 Exclusion criteria include “isolated focal tremors, orthostatic tremor with a frequency >12 Hz, task- and position-specific tremors, and sudden onset and step-wise deterioration.”
7 ET plus also allows presence of mild neurological signs (e.g., resting tremor, impaired tandem gait, or impaired memory).
7 Beyond the simple presence of additional signs captured by the ET plus categorization, it has been suggested that there is prodrome to the ET syndrome, primarily involving nonmotor features.
8 There is much heterogeneity among patients with ET, including age at onset (ranges from infancy to old age), response to pharmacological agents (e.g., alcohol, propranolol), risk factors (e.g., family history, environmental exposures), and speed of progression.
7,9,15Estimating the true prevalence of ET is challenging due to changing definitions, variations in assessment methods, and age distribution.
14 In addition, differentiating ET from normal (physiological) tremor present in all humans can be difficult.
16 A meta-analysis led to a pooled prevalence estimate of 0.9% for ET; however, heterogeneity was noted as a significant limitation.
14 Subgroup analysis of studies that conducted a neurological examination increased prevalence to 2.2%. Age was an important factor. Median crude prevalence estimates increased from 6.3% (age 60–65 and older) to a median of 9.0% (aged ≥80). This effect was also seen in a 45-year (1935–1979) retrospective study that tabulated ET medical chart diagnoses (266 participants aged 2–96 at diagnosis, 58% female) of patients evaluated at the Mayo Clinic and affiliated hospitals.
17 As would be expected, annual incidence rates (per 100,000) increased by decade of age: 2.3 (aged 0–19), 5.4 (aged 20–39), 13.9 (aged 40–49), 34.6 (aged 50–59), 58.6 (aged 60–69), 76.6 (aged 70–79), 84.3 (aged >79). A more recent study estimated ET at 2.2% of the U.S. population by applying age-specific prevalence data based on the three “most methodologically rigorous” population-based studies.
18 In summary, ET is certainly not uncommon, and age seems to be a major risk factor.
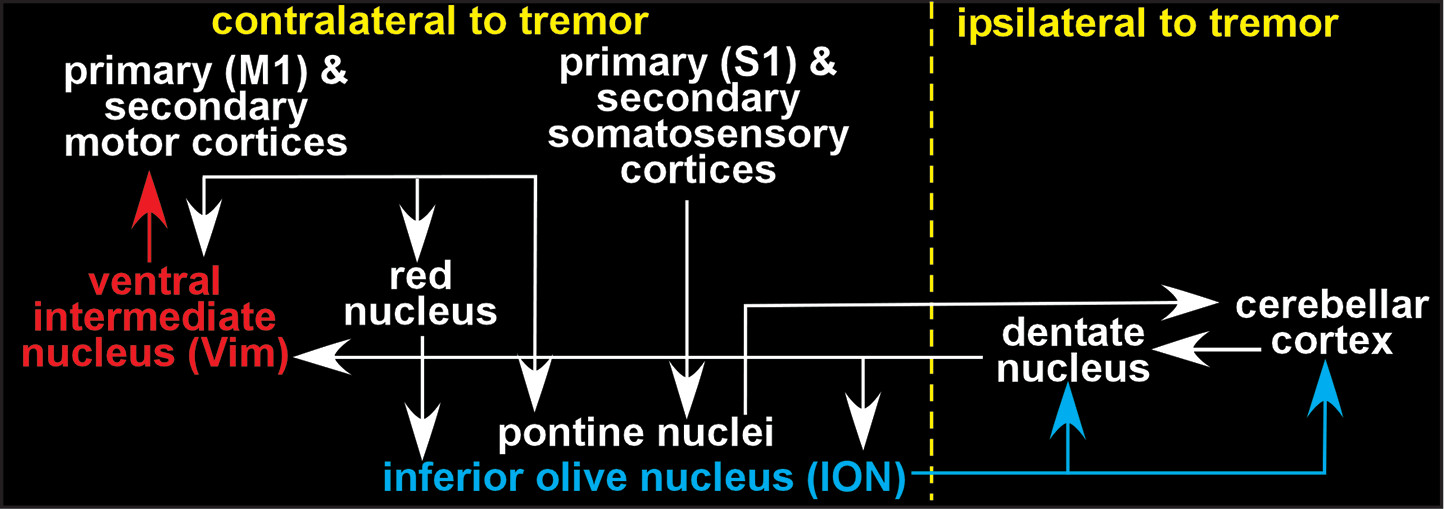
It is generally agreed that the action tremor characterizing ET indicates dysfunction within the motor control network (
Figure 1 and Cover).
1–3 There is much debate regarding which component or components give rise to the oscillatory activity that drives tremor.
1,2,19 The initial proposal was the olivary hypothesis, based on harmaline-induced tremor in animal models, which resembles ET.
20,21 Neurons within the inferior olivary nucleus (ION) have pacemaker properties (i.e., able to produce regular bursts of activity). Harmaline promotes ION generation of rhythmic bursts of activity. ION neurons give rise to climbing fibers, a major input to cerebellar Purkinje cells. Thus, this rhythmic activity is transmitted to the next stages of the motor control network, potentially driving tremor. It has been noted that a toxin-induced tremor in rodents is not necessarily the same as a disease process in humans.
22 On the other hand, there is some evidence that environmental exposure to harmaline-like compounds (i.e., beta-carboline alkaloids) can occur.
23 However, neurons in other areas have pacemaker properties.
1,22 Functional imaging and neuropathology studies have not generally supported the ION as the central generator for ET.
2,19 The cerebellar degenerative model of ET posits that the clinical features (e.g., insidious onset, progressive nature, lack of remission, and increasing prevalence with age) suggest a neurodegenerative etiology. Although both functional imaging and neuropathology studies support cerebellar involvement in ET, there are contradictory findings.
1,2,19,24 One review evaluated research on Lewy body inclusions in the brain stem and deterioration of Purkinje cells.
25 The study concluded that the evidence does not indicate that either of these degenerative mechanisms occurs with more frequency in individuals with ET than in the healthy general population. Another review noted that the alcohol-induced decrease in tremor experienced by many patients with ET does not support the cerebellar degenerative hypothesis.
2 An alternative cerebellar hypothesis is that there is reduced GABAergic tone in ET secondary to changes in the level and/or binding of GABA-A receptors in the dentate nucleus.
1 Another possible central generator for tremor is the ventral intermediate nucleus (Vim) of the thalamus (also called the ventral lateral posterior [VLp]).
1,2 Vim is a major therapeutic target (i.e., deep brain stimulation, surgical ablation), as it contains groups of neurons that can drive tremor (tremor clusters). Alternatively, there is also growing evidence to support the network disorder hypothesis, which posits that pathological tremor results from dynamic changes in network functioning (e.g., hypersynchronization, altered interregional connectivity) rather than being driven by abnormal activity of a single area.
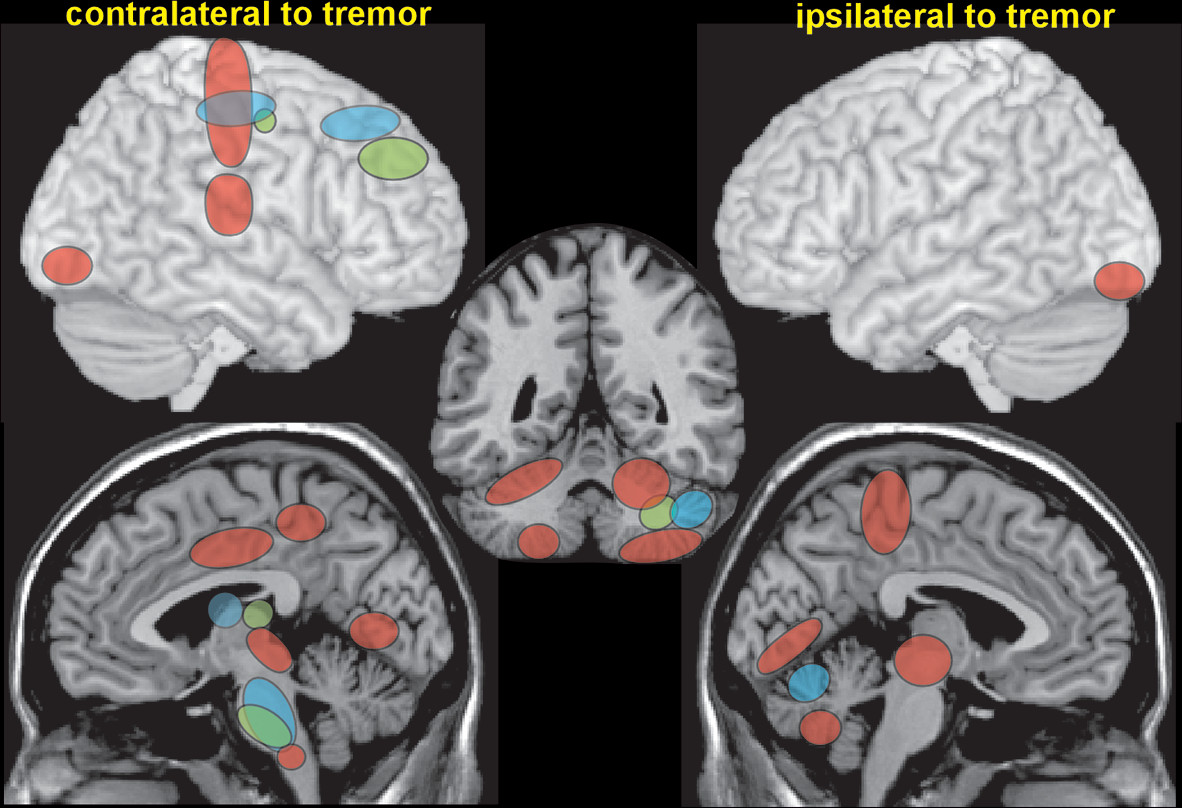
1,2,26Task-activated functional imaging has been used to identify areas that are more active during tremor and therefore may play a role in tremor generation. A functional MRI (fMRI) study utilized electromyography (EMG) of muscle activity power variability at the tremor frequency to identify tremor-related activations in ET patients (
Figure 2).
4 Activations were identified within the motor control network (the primary motor cortex, premotor cortex, primary somatosensory cortex, cerebellum, and brainstem), as well as areas not generally included (the posterior cingulate cortex/precuneus, visual cortices).
4 Coherence and timing between EMG of tremor and magnetoencephalography (MEG) or electroencephalography (EEG) of brain activity have also been used to identify brain areas contributing to tremor generation. Corticomuscular coherence localized to a single area of the motor cortex in two studies that included patients with both early- and late-onset ET (
Figure 2).
5,26 The MEG study used coherence at the tremor frequency between the motor cortex and the rest of the brain (cerebro-cerebral coupling) to identify the tremor-related network (ipsilateral premotor cortex and thalamus, contralateral cerebellum, and brainstem).
5 Another EEG study separated patients with ET into early-onset (average age at onset: 7.6 and 6.8 years) and late-onset (average age at onset: 57.9 years).
6 Corticomuscular coherence localized to the motor cortex, the prefrontal cortex, and the thalamus in both groups but also to the cerebellum and brainstem only in the early-onset ET group (
Figure 2).
Several resting-state (rs) fMRI studies have assessed functional connectivity of parts of the motor control network in patients with ET. A study that segmented the brain into multiple seed regions (91 cortical, 15 subcortical, 26 cerebellar) for a seed-to-voxel based analysis reported that patients with ET had both decreases and increases in functional connectivity compared with healthy individuals.
27 Clinical severity of tremor (Fahn-Tolosa-Marin Tremor Rating Scale, Parts A and B [TRS a+b]) correlated positively with increased connectivity between the cerebellum and thalamus and negatively with decreased connectivity between the cerebellum and sensorimotor cortical areas.
27 Two rs fMRI studies of patients with ET used seed voxels within Vim to assess relationships between tremor severity and functional connectivity of the node of the motor control network that is commonly targeted for treatment of tremor.
28,29 The study that compared ET and healthy groups reported that the areas functionally connected with Vim were generally similar (the primary and supplementary motor cortices, cerebellum, brainstem, thalamus, and basal ganglia).
28 Functional connectivity between Vim and six of the eight small areas that differed between the groups correlated with the clinical rating of tremor severity (TRS a+b).
28 Tremor severity was positively correlated with increased functional connectivity to the motor cortex and negatively correlated with decreased functional connectivity to multiple areas within the cerebellum. The other study also reported that higher functional connectivity of the Vim seed to several areas (the putamen, supplementary motor cortex, and parietal cortex) correlated with greater tremor severity (TRS total score).
29 This study also utilized eigenvector centrality (EC), a network metric of connectivity, to compare general connectivity between the ET and healthy groups. Compared with the healthy group, the ET group had areas of increased EC in the motor cortex and anterior cingulate cortex and decreased EC in the cerebellum, but no difference in EC correlated with clinical tremor severity. Although not in agreement on many aspects, these studies all support the presence of differences in functional connectivity within the motor control network in patients with ET compared with healthy individuals.
As noted above, generation of the oscillatory activity that drives tremor is an area of active investigation. In one study, motor cortex oscillations preceded EMG tremor by approximately 15 milliseconds (ms), suggesting that the motor cortex is driving muscle tremor.
5 In contrast, a study that included dynamic analysis during tremor reported variations in the strength of corticomuscular coherence over time, leading the authors to suggest that the motor cortex does not play a central role in tremor generation.
30 In a study utilizing intraoperative depth recordings, tremor preceded onset of thalamomuscular coherence by approximately 220 ms, indicating that the thalamus is recruited after tremor onset.
31 The authors noted that the length of the delay is consistent with somatosensory feedback via an indirect route driving onset of coherence.
Task-activated studies have reported significant differences between ET and healthy groups in multiple aspects of cerebellar function. One study assessed both cerebellar inhibition of the motor cortex (which occurs via the cerebello-thalamo-motor cortical pathway) and performance of finger movements without visual input (visual occlusion lenses) or with altered visual input (prism lenses).
32 Although there were significant differences between patients with ET and healthy individuals on all tests, no aspect of impaired cerebellar function correlated with clinical tremor severity (TMS total score). An fMRI study that utilized a finger-tapping task reported differences in task-related activations but no differences in performance between patients with ET and healthy individuals.
33 Task-related activations of the dentate nuclei were the only areas that correlated with tremor severity. A later study from the same group reported tremor-related activations in multiple cerebellar lobules and brainstem but not in the deep cerebellar nuclei.
4 A study that assessed coherence prior to and after alcohol ingestion reported that although coherence did not change significantly in the ET group, regression analysis indicated a significant positive relationship between decrease in tremor severity (as quantified by the root mean square of EMG) and coherence localized to the cerebellum.
26 Overall, these results support the importance of frontal motor regions in tremor onset and other tremor-related areas (e.g., the cerebellum) in the modulation of ongoing tremor.
As reflected in recent consensus guidelines, ET is increasingly associated with nonmotor features. One review presents evidence that ET is related to several different profiles of cognitive impairment (global, subcortical, and frontal) and psychiatric symptoms (depression and anxiety).
34 A population-based study (conducted in Spain) found a 1.32 times higher rate of mild cognitive impairment (MCI) among subjects with ET compared with subjects without ET.
35 Mild cognitive deficits in ET are most commonly consistent with a subcortical cognitive profile, encompassing mildly impaired complex attention, executive function, and nonamnestic memory deficits.
36–38 For example, a study of MCI patients (conducted in Korea) found that executive dysfunction was most common in the ET group compared with memory deficits in the non-ET group.
39 A recent study (conducted in the United States) compared ET groups with normal cognition (71%), with MCI (19%), and with dementia (10%).
40 A multiple-domain amnestic MCI subtype was most frequent in the ET with MCI group (54%). Longer duration of tremor was found to be related to an increased risk of MCI.
40 Despite limitations to existing studies (variable definitions of “impaired,” not considering multivariate base rates, and no evaluation of performance validity), amassing evidence usually indicates mild but consistent cognitive impairments in ET. It has also been associated with increased risk of conversion from MCI to dementia.
41Research has also linked elevated psychiatric symptoms to ET. Compared with healthy individuals, ET patients have been found to have higher levels of depression, anxiety, social phobia, insomnia, and fatigue.
42–44 One research group (from India) has assessed relationships between specific symptoms and quality of life (QOL) among patients with ET.
42,45 Greater severity of tremor (TMS a+b), depression, and anxiety correlated with lower QOL, whereas age at onset and duration of tremor did not. Another study (conducted in Latvia) reported a moderate relationship between tremor severity (TMS total score) and social phobia symptoms.
44 A study (conducted in Germany) compared patients with ET receiving outpatient treatment to matched (on age, sex, and TMS a score) community members with previously undiagnosed ET.
46 Although matched on tremor severity, a higher proportion of the treatment group reported tremor-related job impairment (45% versus 25%) and ET-related depressive episodes (71% versus 32%). Thus, emerging evidence points to the value in querying ET patients for nonmotor symptoms.
34Some studies have begun to establish nonmotor phenotypes. Three recent studies compared nonmotor symptoms in patients with ET to patients with Parkinson’s disease (PD).
10,11,47 Two studies reported similar total symptom scores for ET and PD.
10,47 One found a somewhat higher symptom burden for PD compared with ET.
11 Another study reported higher levels of depression and anxiety in both PD and ET patients compared with healthy individuals.
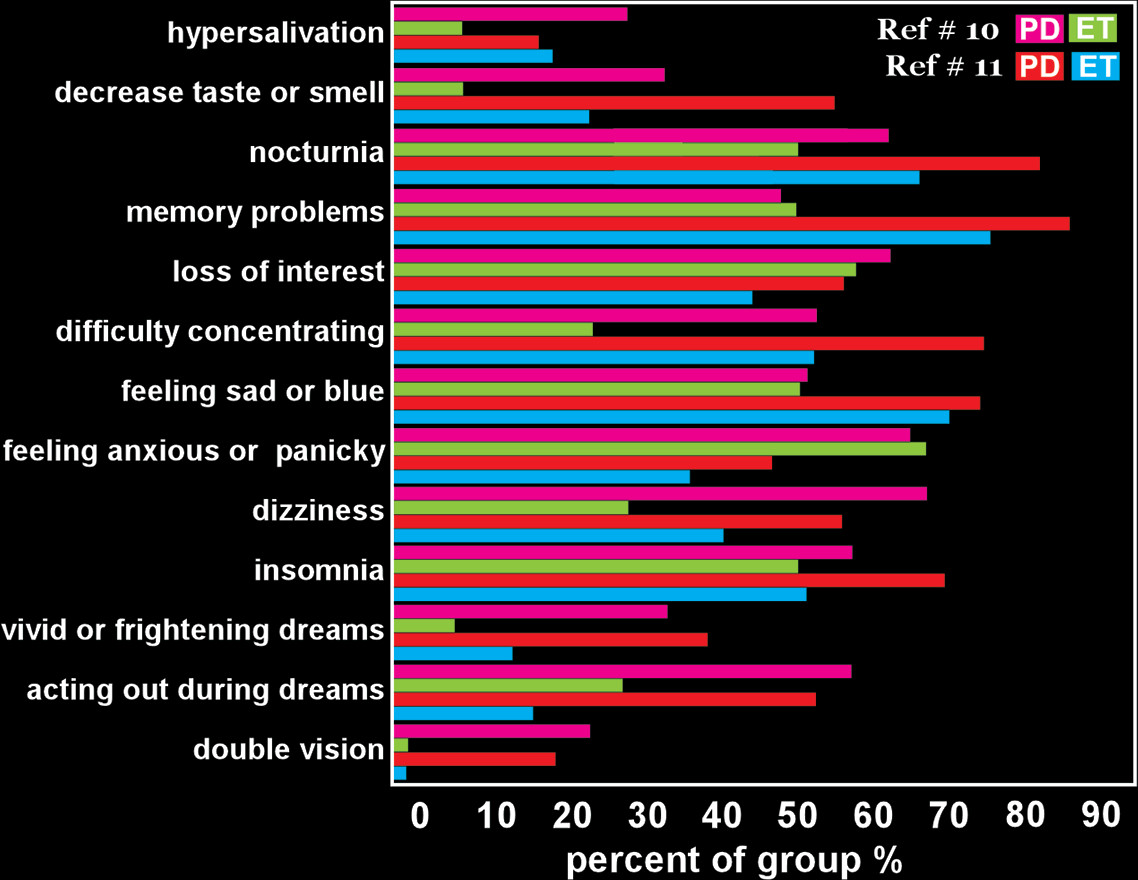
44 These studies highlight the presence of a variety of cognitive, emotional, and behavioral issues in both disorders (
Figure 3). Some symptoms occurred with a higher frequency in the PD groups (e.g., changes in taste or smell, double vision, acting out during dreams, hallucinations). The lower occurrence of olfactory dysfunction in ET may support the value of olfactory assessment in the differential diagnosis of ET from other movement disorders.
48,49Conclusions
ET remains one of the most common motor disorders. Although the mechanism, course, and related features of the disease continue to be controversial, there is increasing evidence that ET is neither benign nor essential. Emerging evidence suggests a neurodegenerative component in some patients with ET. This may be a family of disorders rather than a single homogenous entity. Recent guidelines have incorporated the importance of nonmotor symptoms (e.g., cognitive impairment) by differentiating ET and ET plus. Additionally, there is some evidence for a nonmotor prodrome stage of the disease. With this number of unanswered questions, there remains a significant role for well-designed neuroimaging and assessment studies to elucidate phenotypic differences in patients with ET.