Huntington’s disease is an autosomal dominant inherited, progressive neurodegenerative disorder caused by an expanded trinucleotide extension that codes for mutant huntingtin on chromosome 4.
1 Huntington’s disease is clinically characterized by a triad of symptoms: motor abnormalities, behavioral symptoms, and cognitive deterioration.
2 The formal clinical diagnosis of Huntington’s disease is typically based on the appearance of unequivocal motor signs, even though behavioral signs and cognitive decline often occur before motor signs are present.
2,3 However, with the identification of the exact location of the huntingtin gene, individuals at risk can be tested for the expanded Huntington’s disease gene before any signs and symptoms become apparent. New guidelines have agreed that clinical diagnosis can be made solely on behavioral and/or cognitive signs.
4The behavioral symptoms in Huntington’s disease are diverse
5,6. The most common behavioral symptoms are depressed mood, irritability, and apathy, with a prevalence varying from 33% to 76%, depending on the definition and measurement tools used and disease stage.
6 Of all behavioral symptoms, apathy—defined as “lack of motivation resulting in diminished goal-directed behavior, cognition, and emotion”
7—is the only behavioral symptom that is closely related to disease progression in Huntington’s disease.
5 Therefore, it is suggested that apathy is caused by the neurodegenerative process in Huntington’s disease and could be seen as a marker of disease progression.
8 In Huntington’s disease, apathy is also associated with cognitive dysfunction as well as the use of psychotropic medication.
9In their review of rating scales for the assessment of behavioral symptoms in Huntington’s disease, Mestre et al.
10 discuss three scales for assessing apathy in Huntington’s disease. The Apathy Scale, which is based on the Apathy Evaluation Scale,
13 has been used in some studies.
9,11,12 However, the Apathy Scale has been recommended for screening purposes only. The Apathy Evaluation Scale has been suggested for assessment of the severity of apathy in Huntington’s disease. Mestre et al.
10 favor the clinician version of the Apathy Evaluation Scale due to possible lack of insight among patients with Huntington’s disease.
Previous studies have shown that Huntington’s patients can be unaware of the signs and symptoms of the disease, including behavioral symptoms.
14,15 The degree of impaired awareness of their own disability (anosognosia) varies depending on the symptom and its severity, cognitive function, and disease stage.
15 In clinical trials, researchers often ask a proxy to rate behavioral symptoms to avoid the risk of unawareness among Huntington’s disease gene expansion carriers (HDGECs). However, because it is not always possible to include HDGECs together with a reliable proxy only, it is of great relevance to evaluate whether HDGECs themselves are capable of adequately rating their own severity of apathy using a self-report questionnaire.
To date, only two studies, to our knowledge, have been conducted to investigate the level of agreement between HDGECs and their proxies in rating the severity of apathy by using a self-report questionnaire.
11,16 However, the results of these studies are conflicting. In one study, there was a difference in the reported severity of apathy between clinically diagnosed HDGECs and their proxies,
11 while in the other study, there was no difference in the total apathy score between premanifest and manifest HDGECs and their proxies.
16 These inconsistent results could be ascribed to the different methodology of the studies: the former used the Apathy Scale and included clinically diagnosed carriers only, whereas the latter used the Apathy Evaluation Scale and included both premanifest and manifest carriers. The Apathy Scale is an abridged version of the Apathy Evaluation Scale,
17 and on face value, the two questionnaires are comparable, although with the Apathy Scale there is a lack of psychometric data for the assessment of apathy in Huntington’s disease.
10 Because of the aforementioned conflicting results and the discussed unawareness of apathy among HDGECs, we conducted an additional study to evaluate whether HDGECs are less aware of their apathy severity than their proxies.
Methods
Participants
The REGISTRY study
18 is a European multicenter, longitudinal, observational study conducted in 17 countries. The Leiden University Medical Center is the largest REGISTRY site. All REGISTRY participants at the center seen between January 2013 and August 2014 and their proxies were asked to complete the Apathy Evaluation Scale. The Leiden University Medical Center acquired ethical approval for this study, and all participants provided written, informed consent.
A total of 109 (premotormanifest, N=31; early motormanifest, N=49; and late motormanifest, N=29) HDGECs and their proxies completed the REGISTRY battery and additional questionnaire. All HDGECs were genetically confirmed with a CAG score >39. Carriers with a total motor score ≤5 on the Unified Huntington’s Disease Rating Scale,
19 which indicates no substantial motor signs, were defined as premotormanifest. The group with a total motor score >5 was considered to be motormanifest with obvious Huntington’s disease motor signs. This motormanifest group was further divided into early motormanifest and late motormanifest subgroups according to the disease stage, on the basis of the total functional capacity score.
20 Total functional capacity stages 1 and 2 were considered early motormanifest, and stages 3 and 4 were considered late motormanifest. No individuals classified as stage 5 participated in this study.
Clinical Measures
The Apathy Evaluation Scale was used to quantify the level of apathy. There are three versions of this scale available: one for the patient, one for the proxy, and one for the care professional or investigator. Here, the patient and proxy versions were used. The scale was developed to provide a global measure of apathy on an 18-item questionnaire, rated on a four-point Likert scale with a maximum score of 72.
13 The HDGECs and their proxies were, independently of each other, asked to rate to what degree they agreed with a specific statement (e.g., “she or he gets things done during the day”).
13Statistical Analysis
To assess group differences in demographic and clinical characteristics, we used analysis of variance or the nonparametric counterpart. Because the use of certain medications can affect apathy,
9 the following medication types were identified to have a possible effect on the level of apathy: selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, antipsychotics (atypical and typical), tricyclic antidepressants, bupropion, benzodiazepines, and tetrabenazine. We created a binary variable to indicate whether the HDGECs used any of these medications. To evaluate whether there was a difference among the three groups (premotormanifest, early motormanifest, and late motormanifest) regarding the severity of apathy, we carried out an analysis of covariance (ANCOVA) with medication use and age entered as covariates. We used the Wilcoxon signed-rank test to assess whether gene carriers and their proxies rated apathy severity differently. IBM SPSS, version 23 (IBM, Armonk, N.Y.), was used for all analysis. A significance threshold was set at 0.05, and if multiple comparison was carried out, Bonferroni correction was applied.
Results
The group characteristics are presented in
Table 1. The three groups differed significantly in age (F=33, df=2, 106, p<0.01), total motor score (H=79, df=2, p<0.01), and medication use (i.e., medication use increased from 23% in the premotormanifest group to 65% in the late motormanifest group; χ
2=12, df=2, p<0.01). The groups did not differ in CAG length and gender.
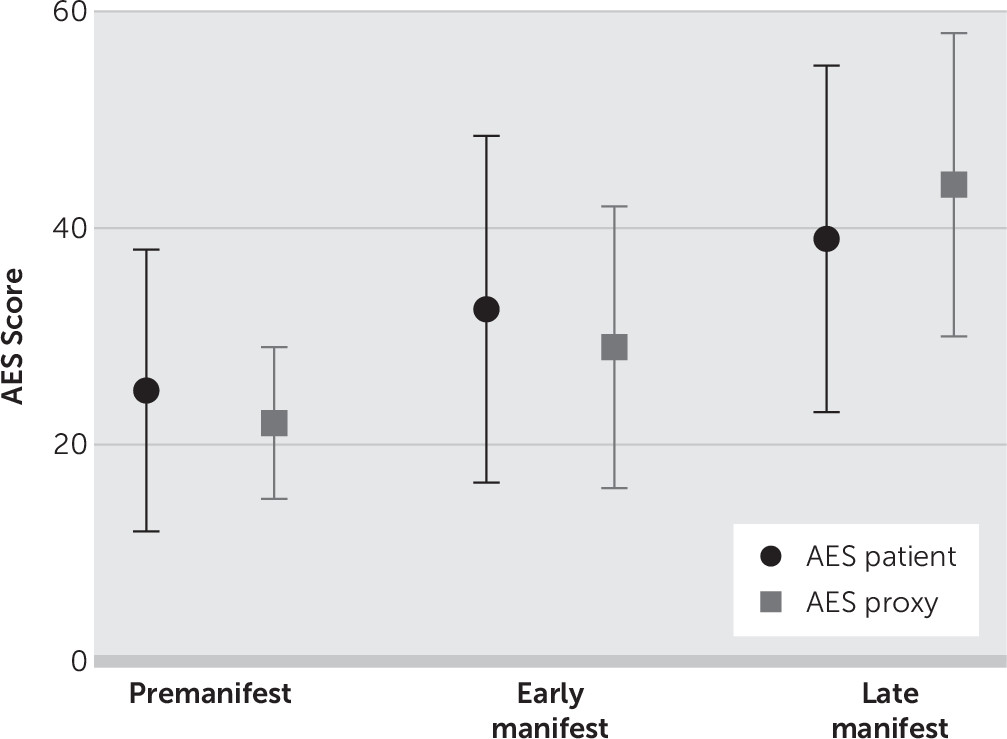
The ANCOVA for the Apathy Evaluation Scale revealed that the three groups differed significantly in apathy score (F=5, df=2, 102, p<0.01). Post hoc analysis showed that the premotormanifest group scored, on average, 10 points lower on the Apathy Evaluation Scale (p<0.01) compared with the late manifest group. The early motormanifest group scored significantly lower on the Apathy Evaluation Scale than the late motormanifest group (mean difference, six points, p=0.03). No significant difference was found between the premotormanifest and early motormanifest groups (
Figure 1).
The Wilcoxon signed-rank test showed that there was no difference in apathy score when the total group of HDGECs was compared with the rating of their proxies (Z=−0.65, p=0.52). However, when the three carrier groups were analyzed separately, the premotormanifest carriers rated themselves as being more apathetic than their proxies (Z=−2.6, p<0.01) (
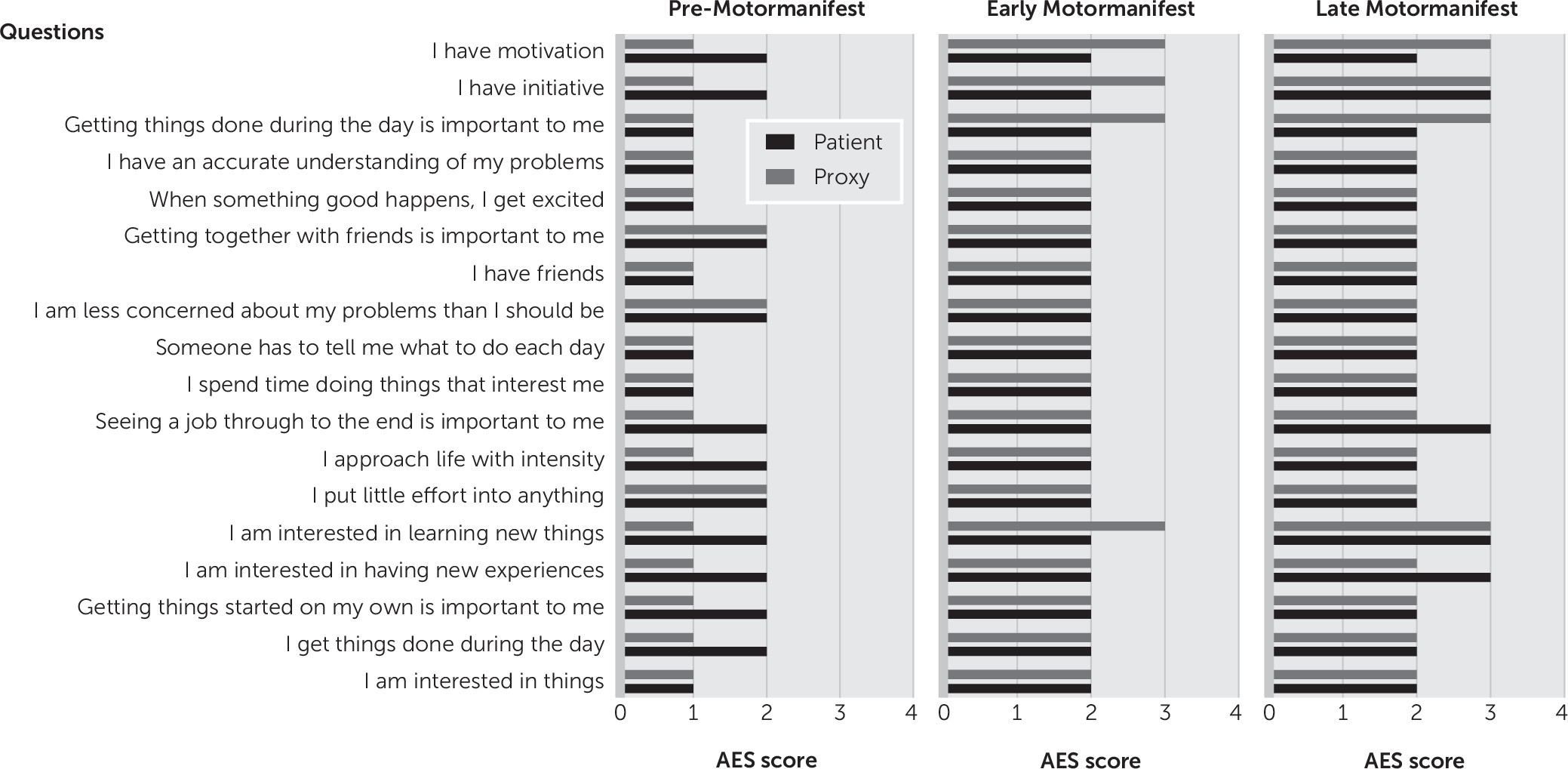
Figure 1). The premanifest carriers rated themselves one point higher (worse) than their proxies on eight of the 18 questions (
Figure 2). In the early and late motormanifest groups, no significant difference was found between the total Apathy Evaluation Scale score of the HDGECs and their proxies. It is noteworthy that the proxies of the early motormanifest group rated apathy on three items one point higher than the carriers themselves, but this did not result in a significantly different total score on the Apathy Evaluation Scale.
Discussion
In our cohort, apathy severity increased overall as the disease progressed throughout the clinically manifest stages. In particular, the premotormanifest and early motormanifest groups scored about the same on the Apathy Evaluation Scale, but there was a significant increase in the score from the early motormanifest stage to the late motormanifest stage. This is in line with previous findings that apathy increases throughout disease progression and that apathy is a common behavioral symptom in the advanced disease stage.
5We did not find a difference in apathy ratings when we compared the score of the entire HDGEC cohort with the score of their proxies, which indicates an agreement on the severity of apathy between gene carriers and their proxies. However, when we evaluated the different disease stages separately here, premotormanifest HDGECs rated themselves higher on the Apathy Evaluation Scale than their proxies, which indicates that premotormanifest HDGECs experienced a higher level of apathy than was noticed by their proxies. These findings are consistent with those of Mason and Barker
16 in that the apathy score of the entire HDGEC group did not differ from that of their proxies and that premotormanifest HDGECs tended to rate themselves as being more apathetic than their companions. However, differences here appear in the evaluation of the motormanifest groups. Mason and Barker
16 reported that early-stage patients also tended to rate themselves as more apathetic, whereas in late-stage disease the proxies scored higher than the HDGECs, which we did not observe. This may be explained by the use of a different definition of the motormanifest groups in our study.
The other study that compared self-reported gene carrier assessment with the caregiver assessment found that the proxies rated apathy as more severe than the carriers.
11 However, in this study, only clinically diagnosed HDGECs were included, and the Apathy Scale was used. The study population was divided according to their cognitive ability: the agreement between HDGECs with good cognitive abilities and their proxies was high, and this agreement weakened as cognitive abilities declined. Because gene carriers with better cognitive function are associated with early disease stage, this group is comparable to the early motormanifest HDGEC group in our study, in which we also found that HDGECs and their proxies agreed on the degree of apathy. However, we did not find that this agreement weakened in the late motormanifest group with assumed declined cognitive abilities.
When we take the results of these three studies together, it seems that in the early stages of disease, HDGECs and their proxies have equal awareness of apathy severity. However, premotormanifest carriers experience more apathy than what is noticed by their proxies. One explanation for this difference may be the hyperalertness of premotormanifest carriers for the development of signs of the disease; the knowledge of being a carrier could lead to a higher report of possible symptoms. This effect may disappear when carriers are clinically diagnosed, as in the early motormanifest stage.
Another possible explanation for this difference is that these apathy symptoms are very subtle,
3,21 and it may be a more internal feeling of which the proxies are not aware. This is supported by the fact that most discrepancies are regarding questions relating to internal drive, such as, “I am interested in having new experiences.” The results of the three studies for the late motormanifest HDGECs are inconsistent, although apathetic patients in advanced stages with cognitive impairments may be less aware of their symptoms.
One limitation of our study is that we may have had a selection bias. We asked all REGISTRY participants during a specific time period to participate in this study. It is possible that individuals who had a greater severity of apathy or cognitive impairment declined to participate. Furthermore, we assumed that the proxy was the most reliable individual to indicate the severity of apathy among the HDGECs. However, the proxy was personally involved and may not have been able to objectively judge the degree of apathy.
In conclusion, we replicated previous findings that apathy is more severe in the advanced stages of Huntington’s disease, and we provided further evidence that HDGECs are capable of assessing their own level of apathy on a self-report questionnaire in the early stages of Huntington’s disease. In particular, the premotormanifest individuals here were aware of subtle changes that were unnoticed by their proxies. Taken together with previous findings, this implies that the absence of a proxy is not a legitimate reason for exclusion in clinical trials for the assessment of apathy in the premotormanifest and early manifest stages of the disease.