The most common neurodegenerative form of parkinsonism is Parkinson’s disease, which is a clinical syndrome characterized by lesions in the basal ganglia, predominantly in the substantia nigra. Parkinson’s disease makes up approximately 80% of cases of parkinsonism, and it is recognized that nonmotor symptoms are prominent (
1,
2). Much current clinical research surrounds the frequency and effect of nonmotor symptoms on patients with parkinsonism, including Parkinson’s disease psychosis, which is common but frequently underrecognized and undertreated (
3). Psychotic symptoms will develop in up to 60% of patients with Parkinson’s disease and in up to 75% of patients with Parkinson’s disease and concurrent dementia (
3,
4).
The care of patients with Parkinson’s disease psychosis is associated with significant healthcare use. In a Medicare survey of claim data from 2000 to 2010, patients with Parkinson’s disease psychosis had higher all-cause costs and resource use (
3). The highest annual cost differentials were found in long-term care costs ($31,178 for Parkinson’s disease psychosis versus $14,461 for Parkinson’s disease without psychosis), skilled nursing facility costs ($6,601 for Parkinson’s disease psychosis versus $2,067 for Parkinson’s disease without psychosis), and inpatient costs ($10,125 for Parkinson’s disease psychosis versus $6,024 for Parkinson’s disease without psychosis). Long-term care use and expenditures were also significantly higher for patients with Parkinson’s disease psychosis, who spent an average of approximately 179 days in long-term care, compared with 83 days for patients with Parkinson’s disease without psychosis.
The presence of psychotic symptoms is not only an independent cost-driving factor but also intrusive to the patient’s daily life and a significant determinant of increased caregiver burden; it sometimes exceeds that imposed by the motor symptoms that are classically associated with Parkinson’s disease (
5,
6). In a community-based Parkinson’s disease sample, minor symptoms of Parkinson’s disease psychosis (e.g., illusions, sense of presence, passage hallucinations) were associated with more depressive symptoms and worse quality of life (
7). The presence of hallucinations and psychotic symptoms are also an independent risk factor for nursing home placement and mortality in Parkinson’s disease patients (
8,
9). When patients with Parkinson’s disease psychosis are admitted to long-term care facilities, their associated disruptive behaviors can have a significant effect on long-term care personnel and other residents.
Balancing control of the motor and psychiatric symptoms of parkinsonism has historically been challenging because of a paucity of evidence-based strategies. Quetiapine is indicated for the treatment of schizophrenia and bipolar disorder; however, it is commonly used off-label for the management of psychosis in parkinsonism. For patients with this condition, quetiapine dosing is titrated from 12.5 mg nightly up to a range of 50–150 mg per day (
10). Quetiapine is similar in structure to clozapine and has antagonist activity at histamine, muscarinic, and serotonin 5-HT
2A receptors, with minimal affinity for dopaminergic D
2 receptor (
11). In the American Academy of Neurology evidence-based practice parameter on the treatment of psychosis in Parkinson’s disease, quetiapine was classified as level C (i.e., possibly effective) (
12). A task force of the International Parkinson and Movement Disorder Society concluded in an evidence-based report that although there is insufficient evidence to make adequate conclusions on the efficacy of quetiapine for the treatment of Parkinson’s disease psychosis, it is nevertheless considered “possibly useful” in practice (
13). A joint task force of the European Federation of Neurological Societies and the Movement Disorder Society–European Section also states quetiapine as possibly useful (
14). Nearly contemporaneous to the publication of these evidence-based statements, the use of quetiapine and other antipsychotics in patients with Parkinson’s disease has been found to be associated with an increased risk of mortality (quetiapine hazard ratio of 2.16 [95% CI, 1.88–2.48] over nonuse) (
15) in a U.S. Department of Veterans Affairs Parkinson’s disease population. In addition, the Food and Drug Administration has approved a new molecular entity, pimavanserin, for the treatment of Parkinson’s disease psychosis, and it has been declared “clinically useful” for this condition (
13). Given these new developments, it is important to reevaluate the role of quetiapine in the treatment of psychosis in patients with parkinsonism.
Methods
Literature Search Strategy and Data Sources
The literature search strategy was designed to identify randomized controlled trials (RCTs) of quetiapine in the treatment of psychosis in patients with parkinsonism. The literature search was performed using these databases: PubMed, Cochrane Central Register of Controlled Trials, and EMBASE. The search used the keyword “quetiapine.” We limited our search to English-language-only articles published from January 1991 to October 2017. Additionally, we manually searched the reference lists of identified publications for additional studies to supplement our electronic search.
Study Selection
Two reviewers (JC, LM) performed the literature research in parallel and independently. Reviewers discussed and selected articles to be included. Studies were included in the qualitative review if they were randomized and controlled with either placebo or an active comparator, enrolled individuals with a diagnosis of parkinsonism, assessed the efficacy of quetiapine on psychotic symptoms, and evaluated adverse effects including motor outcomes. When disagreement arose between screeners on studies to be included in the final synthesis, a third reviewer resolved the discrepancy.
The first level of screening examined the title and abstract of publications for inclusion or exclusion. At the second level of screening, the full-text publications were retrieved and reviewed for inclusion or exclusion. Reference lists were also screened to identify additional studies. At the final level of screening, data were extracted from the final list of RCTs.
Data Extraction and Quality Assessment
Study methodology and patient- and treatment-level data were extracted from full-text publications under predefined headings. Each study underwent quality assessment for risk of bias based on Cochrane metrics (
16). The quality assessment for RCTs systematically addressed seven types of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias.
Results
Studies Identified
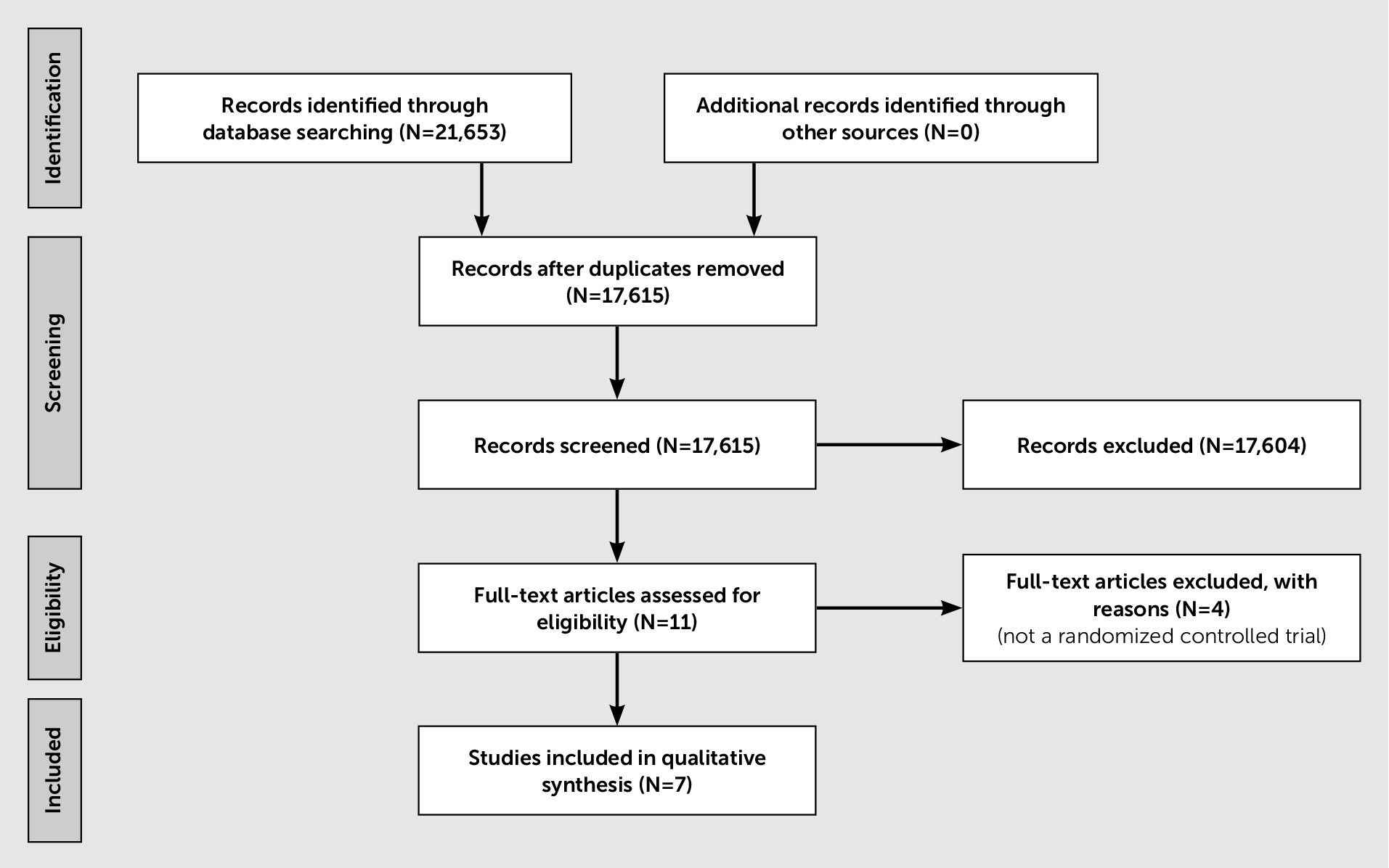
The systematic literature search identified seven studies on the efficacy of quetiapine in treating psychosis in parkinsonism (including Parkinson’s disease psychosis, dementia with Lewy bodies, and dementia with parkinsonian features) (
Figure 1). Five of the RCTs compared quetiapine to placebo (
17–
22), and two RCTs compared quetiapine to clozapine (
23,
24). A summary of the RCT results is provided in
Table 1. The seven unique RCTs included a total of 241 patients with parkinsonism and psychosis. Quetiapine at doses between 12.5 mg and 300 mg per day (mean daily dose of 103 mg) administered from 4 to 22 weeks (mean duration of 12 weeks) failed to significantly reduce psychotic symptoms in participants with parkinsonism compared with placebo or clozapine when objectively assessed on the Brief Psychiatric Rating Scale (BPRS), the most frequently reported scale in these RCTs. In the two clozapine-comparator controlled studies, quetiapine demonstrated no advantage over clozapine in terms of efficacy or adverse effects; in one of the studies (
23), hallucination and delusion scores favored clozapine over quetiapine. Overall, the mean completion rates for all groups were poor, and rates did not differ between participants treated with quetiapine and those who received placebo. Across all RCTs, the completion rates for quetiapine, clozapine, and placebo were 66%, 68.5%, and 66%, respectively. In the two RCTs comparing the efficacy of quetiapine to clozapine, the completion rates were more favorable compared with clozapine (80% and 68.5%, respectively) (
23,
24).
Overall, adverse effects reported for participants treated with quetiapine included confusion, dizziness, headache, orthostatic hypotension, somnolence, and worsening parkinsonism. Although individual RCTs reported quetiapine-associated worsening of parkinsonism, the overall data demonstrate that quetiapine does not significantly worsen motor function as measured by Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores. The systematic quality assessment revealed that all RCTs were subject to high risk of attrition bias due to incomplete outcome data (
Table 2).
Details of the Studies
Fernandez et al. (
17) performed a randomized double-blind placebo-controlled study on the use of quetiapine for patients with Parkinson’s disease and visual hallucinations. Participants were randomly assigned to treatment arms and initiated at 25 mg of quetiapine or matching placebo at bedtime. Dosage increased by 25 mg increments every 3 to 7 days until reaching a maximum dosage of 150 mg, or the complete cessation of nocturnal hallucinations. The primary dependent variable was the length of REM sleep, measured by polysomnography. The quetiapine group increased in the length of their REM sleep (mean increase=13.6 min), and the placebo group decreased in REM sleep (mean decrease=28.3 min), but the difference between groups was not statistically significant. Hallucination severity was self-reported in one item of the BPRS, which presents a series of psychiatric symptoms rated on a 7-point scale in which larger scores indicate greater severity. Although the quetiapine group experienced greater reduction in hallucination severity (mean reduction 1.32 [SD=1.13]) than the placebo group (mean reduction 0.04 [SD=0.82]), p=0.02, the change in total BPRS scores was not statistically different between groups. There were no statistically significant differences between quetiapine and placebo in UPDRS motor scores or in the number of adverse events experienced by either group. The completion rate was only 68.75% (N=11/16), with 50% of the quetiapine group and 87.5% of the placebo group finishing the study. Notable reasons for termination from the quetiapine group were lack of efficacy in treating hallucinations (experienced by two participants); one participant dropped out because of an adverse event (drowsiness).
A randomized double-blind placebo-controlled parallel group study by Kurlan et al. (
18) assessed the effect of quetiapine on agitation and psychosis in individuals with dementia, Parkinson’s disease, or Alzheimer’s disease with Parkinson’s disease features. Quetiapine and placebo groups started treatment at a dosage of 25 mg of quetiapine or placebo. Dosage was increased as necessary, up to an additional 25 mg every 2 days and up to an absolute limit of 150 mg twice daily. Quetiapine efficacy was assessed using the BPRS, and motor function was assessed using the motor subscale of the UPDRS. Statistical analysis failed to find any statistically significant difference between quetiapine and placebo groups on efficacy, adverse events, or change in UPDRS. The study-wide completion rate was 75% (N=30/40). The quetiapine group had 85% completion, and the placebo group had 65% completion. The authors reported that the most common reasons for dropout were concern over placebo (one in each treatment group), effectiveness of medication (two in the placebo arm, one in the quetiapine arm), and adverse effects (three in the placebo arm, one in the quetiapine arm).
Ondo et al. (
19) conducted a double-blind placebo-controlled, 2:1 randomized, parallel trial to evaluate the efficacy of quetiapine in treating hallucinations in patients with Parkinson’s disease. The first 3 weeks of treatment were two doses of quetiapine per day—one in the afternoon, one at night—titrating up to 50 mg per dose. Weeks 4–6 of treatment maintained two doses per day while titrating up to a maximum of 200 mg per dose. Hallucinations were assessed with the BPRS and the Baylor PD Hallucination Questionnaire. On the Baylor PD Hallucination Questionnaire, participants in the quetiapine group had a nonsignificant trend toward improvement (p=0.20). The quetiapine group and placebo had comparable outcomes regarding the change in total BPRS score and change in the score on the BPRS hallucination item. The main adverse events in the drug group were sedation (40%) and subjective worsening in Parkinson’s disease (19%), although there was no statistically significant worsening of parkinsonism on the UPDRS motor scores. Twenty-six out of 31 participants (83.87%) completed the study, with 80.95% completion from the quetiapine group and 80% from the placebo group. Reasons for dropout from the quetiapine group included serious unrelated illness, lack of effectiveness, and noncompliance. The placebo group had two dropouts from unrelated serious illness.
Rabey et al. (
20) performed a double-blind, placebo-controlled RCT on the efficacy of quetiapine on drug-induced psychosis in patients with Parkinson’s disease. All the participants in this study had been successfully treated with dopaminergic medication for at least 2 years before recruitment. Participants had psychotic symptoms (hallucinations or delusions) that significantly affected their quality of life. Participants were randomly assigned to either the quetiapine or the placebo group for 12 weeks of treatment. Both groups began with one 12.5 mg dose of quetiapine or placebo per day. Over a titration period of up to 4 weeks, dosage increased until the psychotic symptoms resolved or side effects became severe. The outcomes experienced by the quetiapine group and placebo group were comparable, and the differences were not statistically significant. There were no statistically significant differences in the UPDRS motor scores from baseline to final point of assessment in either group. The most commonly occurring adverse event was somnolence, observed in seven participants from the quetiapine group and two participants from the placebo group. The study-wide completion rate was 55.17% (N=32/58), with 50.00% completion in the quetiapine group and 60.71% completion in the placebo group. The most common reason for dropout was a lack of therapeutic response, which occurred in 10 of 15 dropouts in the quetiapine group and nine of 11 dropouts in the placebo group. Three participants in the quetiapine group discontinued treatment due to side effects, of which two were due to somnolence.
Shotbolt et al. (
21,
22) conducted a double-blind placebo-controlled RCT to evaluate the efficacy of quetiapine for psychosis in Parkinson’s disease. The study period lasted 12 weeks, during which participants were started on a daily dose of 25 mg of quetiapine or placebo and titrated through the first 6 weeks up to a maximum dosage of 50 mg in the morning and 100 mg at night. The primary dependent variable was the time remaining in treatment at the time of dropout. This outcome was based on the theory that “patients would drop out if their psychosis failed to improve or deteriorated and would stay in if their symptoms were improving” (
21). On average, participants in the quetiapine group dropped out sooner than those in the placebo group, but this difference was not statistically significant. The secondary assessment measures were the UPDRS total score, UPDRS motor score, BPRS, Neuropsychiatric Inventory (NPI), and Baylor PD Hallucination Questionnaire; none of these measures showed statistically significant changes between baseline and final observation. The quetiapine group had three adverse events—all drowsiness—followed by dropout, and the placebo group also had three adverse events—two cases of drowsiness, one case of confusion—followed by dropout.
Clozapine has improved psychosis in Parkinson’s disease in two multicenter, placebo-controlled trials and is commonly used in clinical practice (
25,
26). We identified two RCTs that compared quetiapine to clozapine and met inclusion criteria (
23,
24). Merims et al. (
23) randomly assigned patients with psychosis and Parkinson’s disease to receive treatment with quetiapine or clozapine and assessed outcomes with the delusion and hallucination items of the NPI and the Clinician Global Improvement-Change Scale (CGI-C). Although the hallucination frequency was reduced for both the treatment groups, the baseline-to-final-assessment difference was statistically significant only for the clozapine group. Participants in the clozapine group experienced a statistically significant reduction in the frequency of delusions, whereas those assigned to quetiapine actually increased delusion frequency, but not to a consistent degree. The change in CGI-C scores was comparable between the two treatment arms. The study provides the UPDRS total score but not the motor score; however, the authors did not observe worsening in parkinsonian symptoms (measured with UPDRS) in any of the treatment arms. Only 59.26% of the participants (N=16/27) completed the study: 69.23% of the quetiapine group and 50.00% of the clozapine group. Lack of treatment efficacy was the most common cause for dropout from the quetiapine group, and a decreased leukocyte count was the most common cause for dropout from the clozapine group.
In the second RCT that compared quetiapine to clozapine, Morgante et al. (
24) randomly assigned participants with psychosis and Parkinson’s disease to either quetiapine or clozapine treatment to assess effect on hallucinations, suspiciousness, and hostility. Outcome measurements included the BPRS, Clinician Global Improvement-Severity Scale, Abnormal Involuntary Movement Scale, and the total score of the UPDRS. Although the outcome assessors were blinded to the treatment assignments, the participants were informed about the drug they were receiving. The starting dose was 25 mg per day of quetiapine or 6.25 mg per day of clozapine. Dosage was titrated by a neurologist aware of the treatment conditions, with maximum doses of 200 mg daily of quetiapine or 50 mg daily of clozapine. Posttreatment scores on the BPRS, Clinician Global Improvement-Severity Scale, and Abnormal Involuntary Movement Scale were improved compared with baseline for both the quetiapine and the clozapine groups. Overall, the UPDRS scores remained unchanged; however, a mild worsening of parkinsonism was observed in three patients treated with quetiapine (more than 100 mg per day). The efficacy of both drugs was comparable, as the differences between quetiapine and clozapine groups were not statistically significant. Both groups experienced a small number of adverse events, but no inferential statistics were provided comparing quetiapine to clozapine. The majority of participants (88.89% [N=40/45]) completed the 12-week study: 90.91% of the quetiapine group, 86.96% of the clozapine group. One participant in the quetiapine group withdrew because of sedation, and another participant withdrew as a result of a “confusional state.” Dropout in the clozapine group was attributed to sedation, dizziness, and severe hypotension.
Discussion
The aim of this comprehensive systematic literature review is to qualitatively evaluate the efficacy and safety (including effects on motor function) of quetiapine as compared with placebo or other interventions for the treatment of psychosis in parkinsonism. Our methodology aimed to gather data for all forms of parkinsonism. We found that the published RCT evidence is dominated by investigations of patients with psychosis and idiopathic Parkinson’s disease.
In a network meta-analysis, Iketani et al. (
27) reported that the utility of quetiapine for the treatment of psychosis (assessed by BPRS) in patients with idiopathic Parkinson’s disease is inferior to that of placebo and that use of quetiapine was likely to lead to deterioration of motor function. The meta-analysis by Iketani et al. was confined to RCTs of patients with idiopathic Parkinson’s disease. Additionally, the meta-analysis included only four unique (i.e., nonredundant) RCTs of quetiapine, which comprised a total of 138 individuals. In the present study, we analyzed data from seven unique studies with a total of 241 subjects with parkinsonism and psychosis (including Parkinson’s disease psychosis, dementia with Lewy bodies, and dementia with parkinsonian features) who were randomized to receive either quetiapine or a comparator (placebo or clozapine). Quetiapine at doses between 12.5 mg and 300 mg per day (mean daily dose of 103 mg) administered from 4 to 22 weeks (mean duration of 12 weeks) failed to significantly reduce psychotic symptoms in patients with parkinsonism compared with placebo or clozapine when objectively assessed on the BPRS, the most frequently reported scale in these RCTs. Overall, in four of five placebo-controlled RCTs, quetiapine failed to significantly improve psychosis. In two clozapine-comparator controlled studies, quetiapine demonstrated no advantage over clozapine in terms of efficacy or adverse effects; in one of the studies, hallucination and delusion scores favored clozapine over quetiapine.
Across all RCTs, mean completion rates for all groups were similarly poor. The RCT mean completion rates for quetiapine (66.5%) and placebo (73.4%) were similar, which suggests that dropouts cannot be substantively attributed to quetiapine dose titration methodologies. However, in clozapine-comparator RCTs, the mean completion rates for quetiapine (80%) were more favorable comparerd with clozapine (68.5%). Overall, adverse effects reported among participants treated with quetiapine included confusion, dizziness, headache, orthostatic hypotension, somnolence, and worsening parkinsonism. Despite reports of worsening parkinsonism, the overall data demonstrate that quetiapine does not significantly worsen motor function as measured by UPDRS motor scores. The regularity of discontinuations due to adverse effects associated with quetiapine in participants with psychosis and parkinsonism indicates that use of quetiapine in this population may not be as well tolerated as traditionally believed. The most common adverse effects leading to quetiapine discontinuation were confusion and somnolence.
In 2008, a task force of the International Parkinson and Movement Disorder Society published a critique of rating scales for the assessment of psychosis in Parkinson’s disease and recommended four scales for use in clinical studies: the BPRS, NPI, Positive and Negative Syndrome Scale, and the Scale for Assessment of Positive Symptoms (
28). The task force labeled these scales as “recommended” with the caveat that none contained all the basic content and mechanistic and psychometric properties to be deemed adequate for capturing the entire phenomenology of Parkinson’s disease psychosis and that none have undergone formal psychometric evaluation in patients with psychosis and Parkinson’s disease. Given the limitations of the recommended scales, the task force also recommended using the CGI-C as a secondary outcome scale to complement the more detailed psychosis rating scales. We found that of the seven RCTs in our study, six used the BPRS as a primary outcome and all used the CGI-C as a secondary outcome. This is consistent with the task force recommendations and represented best practice at that time for clinical research in psychosis with Parkinson’s disease. Despite this, the RCTs failed to reveal any statistically significant differences between quetiapine and placebo or clozapine for efficacy.
The failure to detect a statistically significant difference could also have been due to underpowering of the RCTs and the overall poor completion rates for treated and placebo groups in some studies. Additionally, none of the psychosis rating scales used in the quetiapine RCTs (i.e., Baylor PD Hallucination Questionnaire, BPRS, NPI) have undergone psychometric evaluation in the parkinsonism population (
28,
29); it is possible that the lack of significant differences between quetiapine and placebo or clozapine may be due to issues of instrument reliability, validity, or sensitivity. Given the psychometric limitations of the rating scales used in the seven RCTs, we believe that performing a quantitative analysis (i.e., meta-analysis) would only have yielded quantitatively indeterminant results and would not substantively alter any clinically relevant conclusions reached by our qualitative study.
Quetiapine has traditionally been widely prescribed for the treatment of psychosis in patients with Parkinson’s disease as a result of clinical impressions of efficacy and tolerability. The use of quetiapine, unlike clozapine, does not require mandated laboratory monitoring and, unlike other antipsychotics, is not generally associated with worsening of motor function. Generally, quetiapine is considered to be well tolerated. We found that the RCT completion rate among participants treated with quetiapine was no better than the completion rate among those who received placebo. However, completion rates were better than the rates among participants treated with clozapine. Additionally, the psychosis rating scales used to measure efficacy outcomes in the quetiapine RCTs have not been deemed psychometrically suitable for this use. Given our negative findings and the emerging data of increased mortality in Parkinson’s disease patients treated with quetiapine and other antipsychotics, as well as the recent introduction of a novel antipsychotic pimavanserin for treatment of psychosis in Parkinson’s disease, the overall treatment landscape for psychosis in parkinsonism has evolved to a point at which clinicians must recalibrate the traditional clinical impression of quetiapine efficacy and tolerability for psychosis in parkinsonism.
Conclusions
Quetiapine has not demonstrated better efficacy compared with placebo or clozapine for treatment of psychosis in parkinsonism. The RCT completion rates among quetiapine-treated participants was poor. Confusion and somnolence were the most frequently reported intolerable side effects. On the basis of novel data, clinicians should reevaluate traditional viewpoints on the benefits of quetiapine for psychosis in parkinsonism
Acknowledgments
The authors thank Stephanie Tashiro, M.P.H., for assistance with the literature search process.