Major neurocognitive disorders (dementias) are expected to double worldwide over the next two decades (
20). Alzheimer’s disease (AD) is the most commonly encountered in clinical practice, followed by vascular dementia (
21). It is estimated that by 2050, 13.8 million people will be living with dementia due to AD (
22). In the absence of disease-modifying therapies, the possibility of delaying or preventing dementia has become an important focus (
1–
6). There is a growing consensus that addressing potentially modifiable risk factors is essential to caring for the cognitive health of an aging population. The neurobiological underpinnings of “brain health” approaches will become increasingly important for clinicians to understand. Some of the potentially modifiable factors and their physiologic impact will be reviewed. These include sleep, physical activity, and diet.
Multiple groups now conceptualize the brain and body as a complex nonlinear system (ecosystem) optimized to respond effectively and appropriately (allostasis, adaptive homeostasis, adaptive calibration, resilience thinking framework) to internal and external challenges (stressors) (
2,
23–
26). A stressor is not necessarily intrinsically good or bad, as many are beneficial at one dose level and detrimental at another. The physiologic changes associated with the stress-response may aid in survival when a potential predator is seen. In contrast, when that stress-response is chronic and excessive (e.g., anxiety disorders), there can be deleterious effects (e.g., allostatic load). In addition, individuals interface with stressors in different and distinct ways. Known influences include underlying genetic predisposition, temperament, and prior life experiences. Under normal circumstances, flexible adaptation to challenges maintains the internal environment in a healthy range (allostasis). Severe and/or chronic challenges (high allostatic load) may degrade capacity to cope effectively when a new challenge presents. If not corrected, this may lead to the development of disease (allostatic overload).
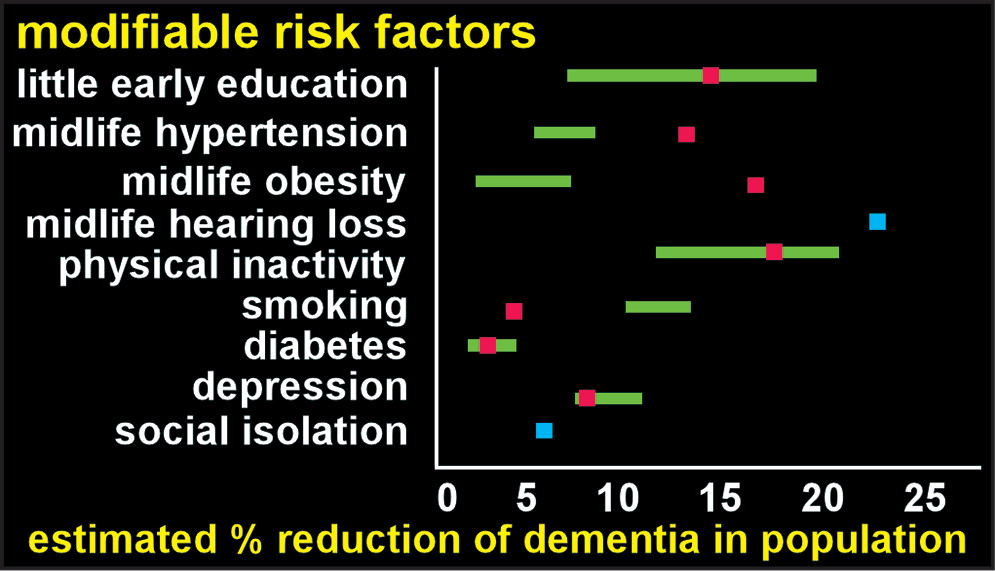
Neurodegenerative dementias typically afflict individuals later in life. However, epidemiological studies have identified multiple early, mid, and late-life factors that alter risk (
8–
11). Early-life exposure to education and enrichment appears to be a protective factor, independent of ultimate occupational attainment (
27,
28). Treatment of acquired sensory impairments (e.g., hearing loss, cataracts) may slow the rate of cognitive decline (
29,
30). Sensory loss may promote social isolation, which is independently associated with an increased risk of dementia (though cause and effect determinations cannot be drawn from epidemiological studies) (
31,
32). Physical activity is associated with lower risks of dementia and depression (
33–
35). Poor sleep quality, a common complaint across the lifespan, has multiple detrimental effects on brain health (
36). Conditions such as metabolic syndrome and smoking are clearly associated with increasing risk of dementia and represent important and well-established targets for prevention (
5). Several recent studies have estimated the population impact of eliminating specific risks (
Figure 1) (
7–
9). Many of these modifiable factors are interdependent. For example, physical inactivity is also associated with increased risk of hypertension, obesity, depression, and diabetes (all risk factors for dementia) (
33,
37,
38). Reported combined risk (after correction for nonindependence) ranged from 28%−48%, indicating substantial potential for reducing the population burden of dementia (
7–
9). Many of these factors have been incorporated into the various “brain health” approaches to these disorders (
1–
3,
5). While evidence in the form of randomized, placebo-controlled trials is rare, there is robust support in the preclinical and clinical spheres (
6).
The system that maintains the brain microenvironment in an optimal state by delivering needed substances (e.g., nutrients, gases, signaling molecules) and removing wastes is an important focus (
39–
41). The cerebral vasculature is one important part of this system, as an intact blood-brain barrier is essential for tight control of movement of substances (e.g., carrier mediated transport, transcytosis) (
42,
43). Glia also contribute to maintaining an optimal brain microenvironment both by secretion of neuroactive compounds and by removal of wastes (i.e., uptake and degradation of toxic substances and debris) (
44,
45). Circulation of CSF via a perivascular route is also important for delivery and removal of substances, although many aspects continue to be actively debated (
19,
42,
46–
50). One major area of contention is whether movement of fluid and solutes through the extracellular spaces is purely passive (diffusion) or involves an active process (convective flow from the arterial to the venous perivascular spaces). The participation of astroglial aquaporin-4 (AQP4) water channels in fluid flow is also contentious. Recently, identification of lymphatic vessels in the meninges has occurred. Preclinical studies suggest that these lymphatic vessels contribute to clearance of wastes, including amyloid beta (Aβ), from the brain to the cervical lymph nodes (
16,
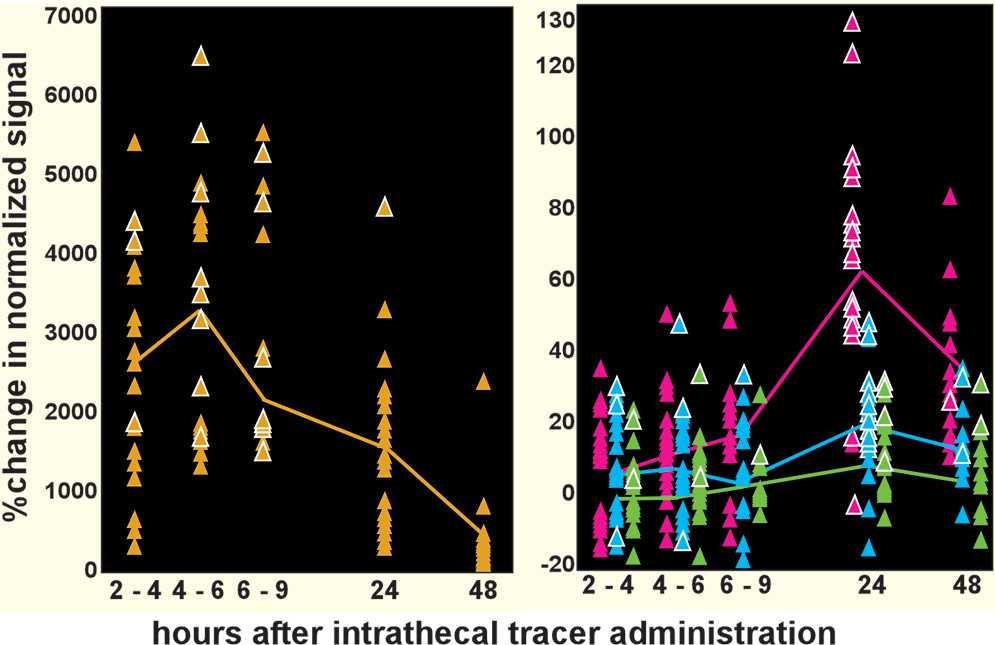
51). Although most studies are preclinical, several important aspects have now been demonstrated in human studies. The passage into the brain of a CSF-borne tracer and its clearance to cervical lymph nodes has been confirmed by sequential MRI (
Figure 2) (
12,
14,
15,
52,
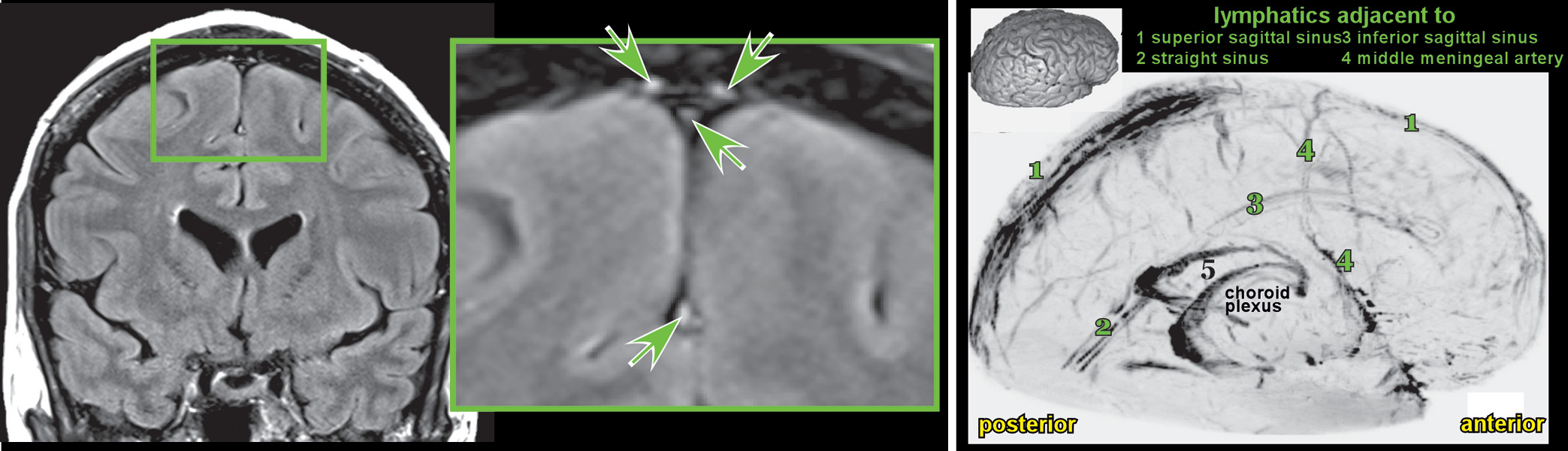
53). The presence of lymphatic vessels in human meninges have been confirmed by both in vivo MRI (
Figure 3) and postmortem microscopy (
16,
54). Modifiable factors that can influence some aspect of this system are potential targets for interventions, some of the more established of which are discussed below (
39).
Abnormal protein aggregation and deposition is a core feature of numerous degenerative illnesses in all organ systems, including many affecting the brain (
39). Multiple protein aggregates are associated with neurodegenerative dementias (e.g., Aβ, microtubule associated protein tau, transactivation response-DNA binding protein 43 kDa, alpha-synuclein, prion protein, huntingtin protein) (
55). The exact role of each of these proteins in neurodegeneration remains in question, as only some correlate highly with symptom presentation and cognitive decline (
56). While a minority of these neurodegenerative proteinopathies have clear genetic mutations, the majority present sporadically. There may be shared physiologic alterations that lead to abnormal protein deposition (e.g., enhanced mis-folding/aggregation, reduced clearance). If impaired clearance is a central pathologic feature early in the development of dementia, then understanding the various factors that affect clearance is critical to understanding prevention strategies.
Sleep difficulties are present in multiple neurodegenerative dementias (
57–
60). The majority of evidence for causal contributions to neuropathologic changes comes from AD (
60). Impaired sleep has been implicated in both the pathophysiology of AD and as a symptom of AD (
61–
64). There is emerging evidence that tau hyperphosphorylation appears very early in regions of the brain that are responsible for sleep-wake cycle regulation (
57). Overnight Aβ production in cognitively healthy individuals is increased by sleep deprivation (
62,
65). Two longitudinal studies of aging in community-dwelling participants reported that presence of excessive daytime sleepiness predicted increased amyloid accumulation (Pittsburgh compound B positron emission tomography imaging) (
61). A cross sectional study in cognitively healthy older adults found that the relationship between self-reported impaired sleep and amyloid burden was mediated by AQP4 genetic variation (
66). Certain AQP4 variants have been associated with more rapid decline in patients with AD, while others have been associated with a slower rate of decline (
67).
In preclinical studies (mouse), locus coeruleus degeneration (an area important for wakefulness) appears to promote Aβ pathology (
68). Preclinical models of AD also show a diurnal variation in Aβ levels, with higher levels present during wakefulness and lower levels during sleep (
69,
70). Clearance of substances including Aβ is enhanced during states of decreased arousal (e.g., sleep, anesthetic-induced suppression of norepinephrine release) (
71–
77). Thus, it is possible that sleep disruption may lead to impaired waste clearance of pathologically relevant proteins, and that deposition of those same proteins may lead to further sleep disruption. If so, the positive feedback loop set up may be difficult to interrupt after a critical threshold is crossed (
71).
There is a clear association between physically active lifestyles and a reduced relative risk of cognitive decline (
34). However, the evidence for exercise as an intervention in older adults has been mixed. A recent review noted that only some studies reported beneficial effects on sleep and depression (
78). Two well-conducted randomized trials found no cognitive benefit in either healthy older adults (
79) or patients with mild to moderate AD (
80). In Parkinson’s disease the benefit of exercise in patients already experiencing symptoms is more promising (
81). In mice, voluntary exercise has been shown to improve both influx and clearance of substances, resulting in less Aβ accumulation and reduced neuroinflammation (
82,
83). In mouse models of AD, exercise was able to reduce pathology including preventing the deposition of Aβ triggered by a high fat diet (
84,
85). Other beneficial effects include increases in neurotrophic factors and neurogenesis (
78,
85). The differences in outcomes across clinical and preclinical studies suggest that the type, timing, and chronicity of exercise are all likely to be important.
The clinical evidence for dietary interventions in AD is reasonably robust. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet has been shown to reduce AD risk with only moderate adherence, whereas the Mediterranean diet (MeDi) appears to require high adherence (
86,
87). In a 3-year study, the MeDi was associated with a better AD biomarker profile in those with higher dietary adherence (
88). Another dietary approach to AD targets impaired brain glucose metabolism, which may occur decades before the disease is clinically evident (
89). Induced hyperglycemia may improve memory in AD (likely mediated through effects on the insulin response), although maintaining a hyperglycemic state is an untenable treatment strategy (
89,
90). Ketone bodies (produced either through a ketogenic diet or ingestion of medium-chain fatty acids) provide an alternative energy source for the brain and have some evidence of cognitive benefit (
89,
91–
95). The initial enthusiasm for simply supplementing a “regular” diet with medium chain fatty acids has been tempered due to recent failure in a phase 3 trial (
https://www.reuters.com/article/us-accera-study-idUSKBN1671LG). Dietary change aimed at increased ketosis may be more effective. The beneficial effects of carbohydrate-restriction on risk factors for dementia (e.g., metabolic syndrome, diabetes) are supported by multiple studies (
96). Recently, a 12-week intervention that included a ketogenic diet, high intensity interval exercise, and memory training in a patient with comorbid mild cognitive impairment and metabolic syndrome resulted in improved cognition and metabolic profile (
97). The potential mechanisms of a ketogenic diet include rescue from the hypometabolic state, reduced oxidative stress, and anti-inflammatory effects (
91,
92,
96,
98). The role of diet is likely complex and intersects with other factors that are independently important in AD pathogenesis such as sleep and metabolic factors (
99).
In conclusion, there is emerging evidence that deceasing the rate and/or increasing the age of onset for neurodegenerative dementias may be possible. A central concept is that an increased toxic load can occur within the brain due to increased production and/or insufficient clearance. Efforts to understand this production and clearance have led to a focus on possible modifiable risk factors and their contributions to this process. Some of those factors may include sleep, physical activity, and diet. A population health approach to preventive efforts, focusing on early-, mid, and late-life modifiable factors, offers a potential roadmap for risk reduction.