Parkinson’s disease (PD) is a neurodegenerative disorder that affects about 1.5% of individuals worldwide (
1). Individuals with PD commonly have speech and vocal problems (
2), including dysphonic speech, reduced loudness, and loss of articulation, which limit their ability to participate in spoken communication and social interactions. Dysphonia in patients with PD worsens during disease progression, with a poor and variable response to pharmacological (
3) or neurosurgical treatments (
4). Indeed, surgical intervention, such as deep brain stimulation, has not been shown to have any definitive positive effects on speech dysfunction (
5,
6). Sewall et al. (
7) found that vocal cord injection may improve Parkinson’s dysarthria, but this result was based on small samples, and the technique the authors used was innovative.
In the 2006 guidelines for PD (
8), speech and language therapy (SLT) was recommended for speech problems in patients with PD on the basis of two randomized controlled trials (RCTs) with 41 participants. In recent years, several RCTs have demonstrated that the behavioral treatment techniques of SLT may be more effective than other treatment techniques in improving the sound pressure level (SPL), Voice Handicap Index (VHI) ratings, and semitone standard deviation (STSD) among patients with PD with speech impairments (
9–
17). In 2012, a systematic review and a meta-analysis in the Cochrane database indicated that no conclusions could be drawn regarding the efficacy of SLTs for speech problems in patients with PD because of a small number of patients, methodological flaws, and the possibility of publication bias (
18,
19). A systematic review and meta-analysis published in 2015 concluded that speech therapy was effective for patients with PD with dysphonia (
20). However, this analysis included only relevant English articles, with few RCTs, and the long-term benefit of speech therapy was not identified. Subsequently, several RCTs have evaluated the effects of SLTs (
9,
11,
12,
16), although these studies have yet to be summarized within one review.
Accumulating evidence in the guidelines and in recent studies provides a rationale for the pooling of current data. The objective of the present systematic review and meta-analysis was to assess the effects of SLTs on dysphonia among individuals with PD.
Methods
Inclusion and Exclusion Criteria
The literature search was limited to RCTs. All included studies met the following criteria: all participants with a diagnosis of PD >18, and the study investigated the effect of SLT on voice and speech disorders. The primary outcomes consisted of SPL and VHI, and the secondary outcomes included STSD, fundamental frequency, and forced vital capacity. Studies that met one of the following criteria were excluded: no outcomes described, no control groups, or animal experiments.
Search Strategy
Two reviewers (H.X., M.L.) independently searched databases, including PubMed, Embase, Chinese National Knowledge Infrastructure, China Science and Technology Journal Database, Chinese Biomedical Literature Database, and Wanfang Database, using the following free-text terms and medical subject heading terms from inception to October 20, 2018: ‘‘Parkinson’s disease,’’ ‘‘dysarthria,’’ “speech,” ‘‘language,’’ “voice,” “treatment,” and “RCT.” In addition, we manually searched the reference lists of reviews and retrieved articles to identify the studies missed by our original search strategy. The search was limited to clinical studies published in English or Chinese.
Data Extraction
Two authors (Z.B., D.L.) extracted useful information independently, and any disagreement was resolved by discussion until an agreement was reached; a third author (Jinpin Li) was consulted if needed. The extracted data included the first author’s name, the publication date, the country or ethnicity of study participants, the number of patients, sex, age, severity and duration of disease, intervention, treatment course, outcomes, and follow-up period in both treatment and control groups.
Quality Assessment
We assessed the risk of bias of the included RCTs using the Cochrane Collaboration’s risk of bias assessment tool.
Statistical Analysis
Meta-analysis and descriptive analysis were performed with the statistical software RevMan 5.3, provided by the Cochrane Collaboration Network. The weighted mean difference was used for the same continuous variables in the result measures with a confidence interval of 95%. Comparisons between groups were performed by using the chi-square test for categorical variables, t test for quantitative variables with normal distribution, and the Mann–Whitney U test when a nonparametric test was required. The statistical heterogeneity of the included studies was evaluated with the chi-square test and the associated I2 value. The test level was set at an alpha of 0.10. If there was no statistical heterogeneity (p>0.1, I2<50%), the fixed-effects model (FEM) was used for the meta-analysis; otherwise, the random-effects model (REM) was used. A p value <0.05 was considered significant.
Results
Study Selection and Characteristics
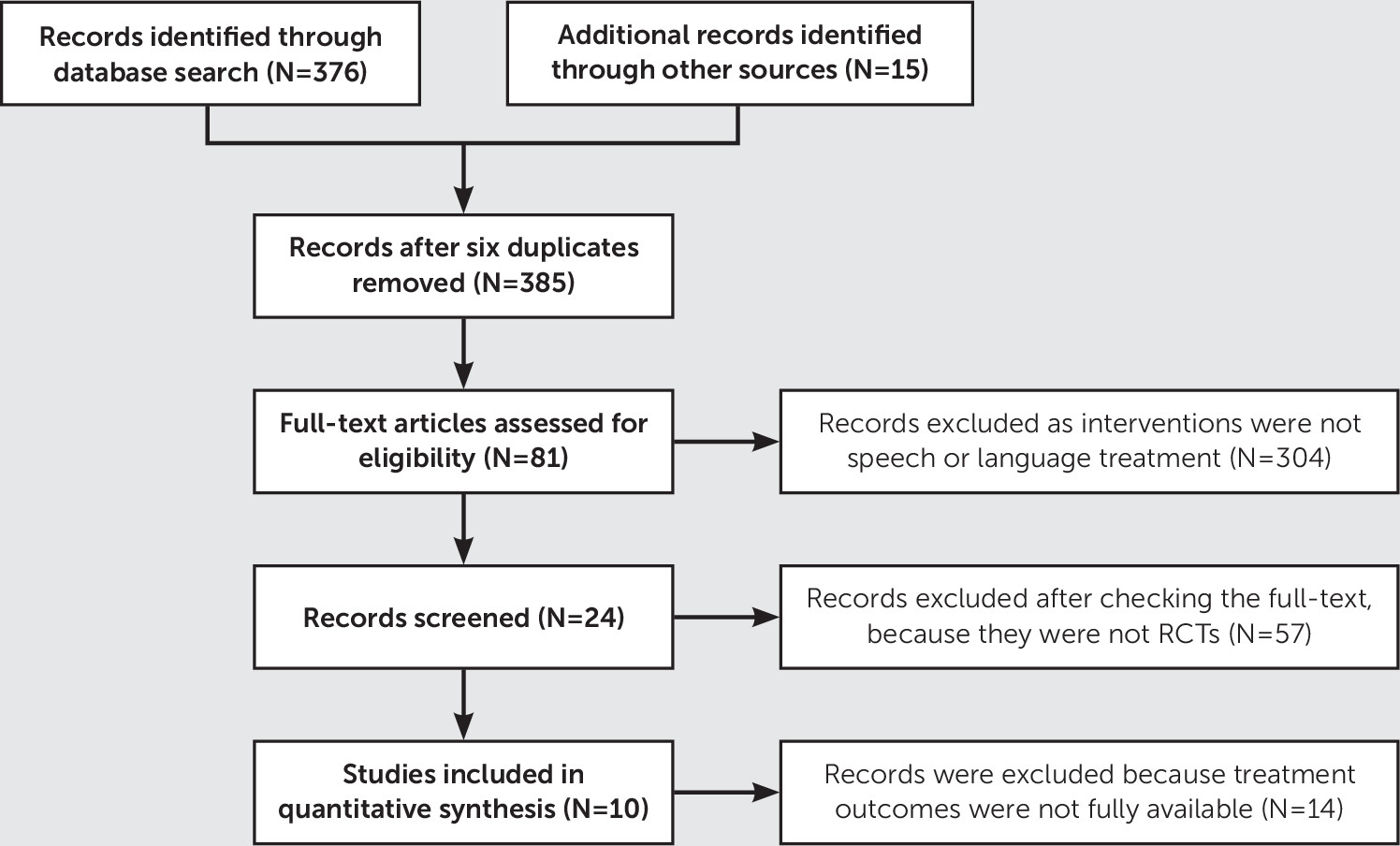
A total of 391 records were identified through database searches and other sources. Among these records, six duplicated records were removed, 304 records were excluded because speech or language treatment was not used as the intervention strategy, 57 records were excluded because full-text checking indicated that they were not RCTs, and 14 records were excluded because treatment outcomes were not fully available. A total of 10 studies were included in this meta-analysis (
Figure 1). (For details on the quality assessment [risk of bias] of the 10 studies, see Table S1 in the
online supplement.)
All of the 10 included studies were RCTs. The demographic and clinical features of the included patients are presented in Table 1. A total of 435 patients (females, N=157; males, N=278) were included in these studies; 230 patients comprised the treatment groups, and 205 patients comprised the control groups. Severity of speech and voice disorders before treatment were assessed by using speech/voice severity ratings or Hoehn-Yahr clinical stages on a scale of 1–5: 1=mild, 2=mild/moderate, 3=moderate, 4=moderate/severe, and 5=severe. Clinical stages of most participants were classified as 1–3. Most participants in the SLT group were treated with Lee Silverman Voice Treatment (LSVT). In only one study were patients treated with prosodic exercises with visual feedback; placebo or no intervention was administered in the control group (
10). LSVT and respiratory effort treatment (RET) both focused on improving vocal loudness. LSVT led to critical improvements, but RET alone was insufficient (
13,
14,
21). Therefore, RET was often considered “placebo treatment” in clinical trials and was compared with intensive speech therapies, such as LSVT (
14,
21–
23). The follow-up periods included 1 month, 6 months, 7 months, 12 months, and 24 months.
Study Quality
All 10 included studies were placebo-controlled RCTs (Table 1). Eight were published in English (
9–
11,
13,
14,
15,
17,
21) and two in Chinese (
12,
16). We assessed the risk of bias with the Cochrane risk of bias assessment tool. Most studies were of moderate quality. Although participants were randomly assigned to interventions, the methods of sequence generation allocation concealment and blinding of participants and outcome assessors were unclear in most of the included studies.
Outcomes of Meta-Analyses
SPL.
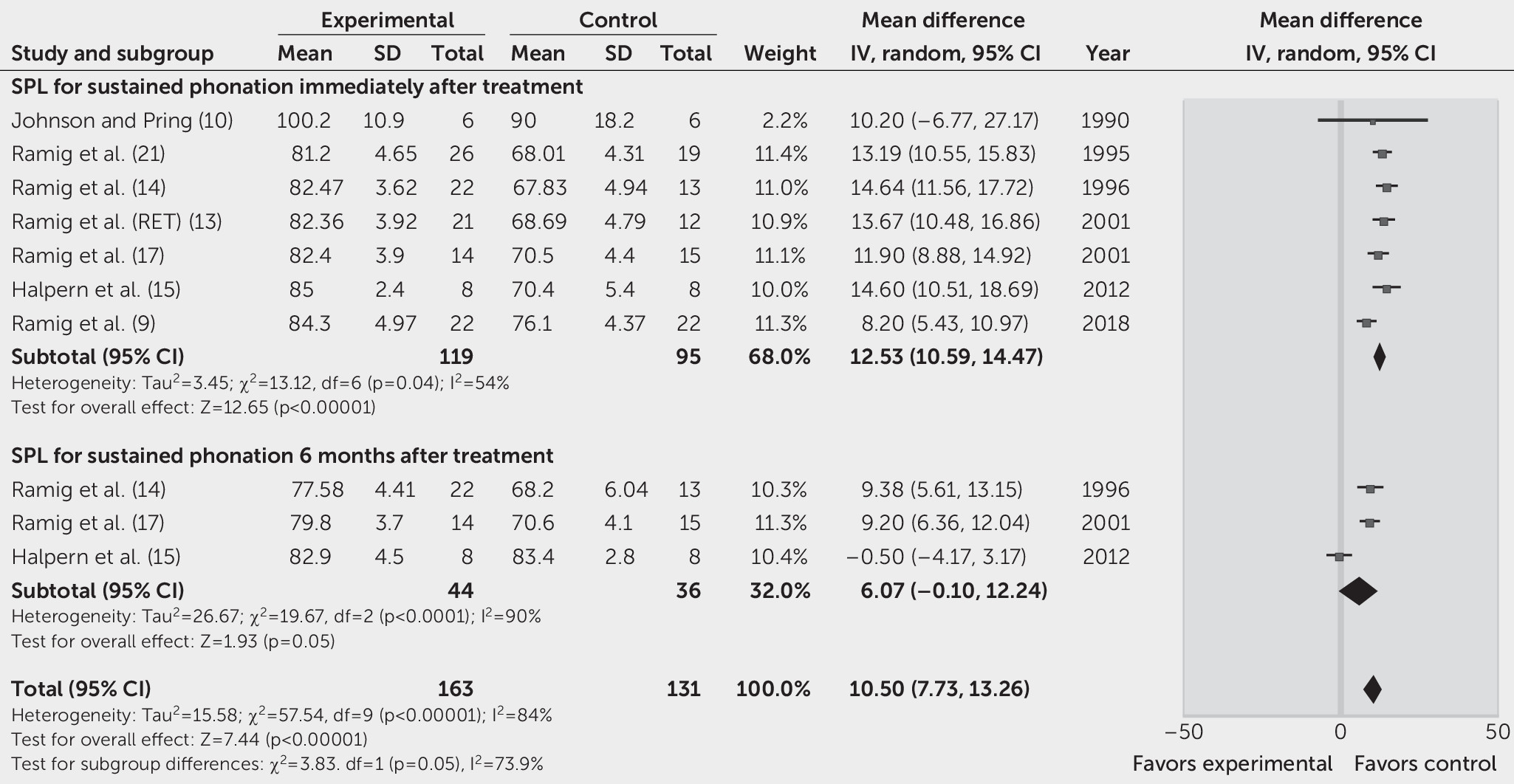
Seven studies reported the SPL of sustained phonation as the primary outcome (
9,
10,
13–
15,
17,
21). Immediately after SLT (1 month or 4 weeks), an increase of 12.53 dB (95% CI=10.59, 14.47, p<0.00001; REM, I
2=54%,) in the SPL was observed in the treatment group, compared with the control group (
Figure 2).
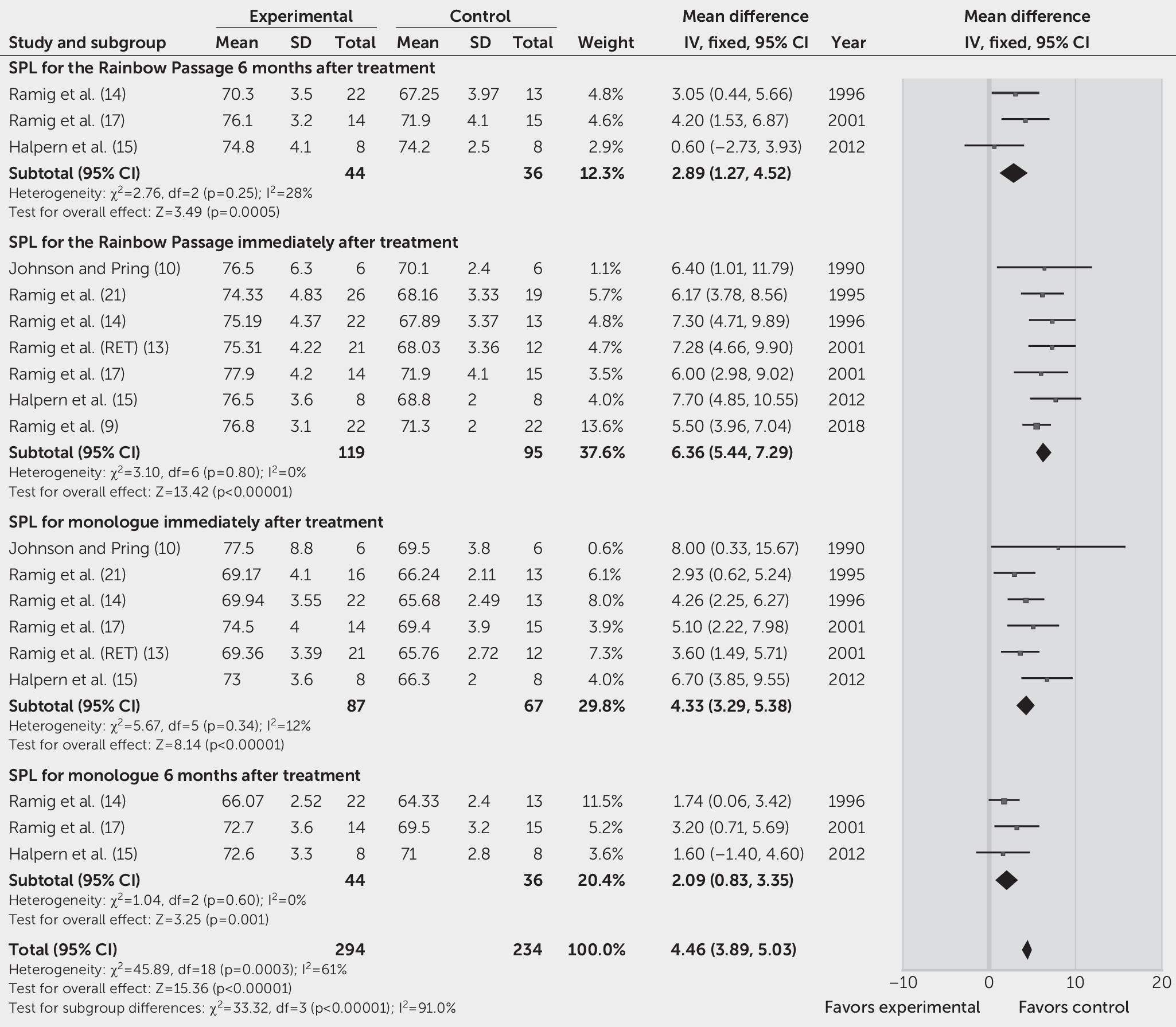
Seven studies reported the SPL during reading of the Rainbow Passage as the primary outcome (
9,
10,
13–
15,
17,
21). FEM analysis showed that immediately after SLT, the SPL score increased by 6.36 dB in the SLT group, compared with the control group (95% CI=5.44, 7.29, p<0.00001; I
2=0%) (
Figure 3). The pooled estimate of the effect of SLT on the SPL of monologue from six studies was 4.33 dB (95% CI=3.29, 5.38, p<0.00001; I
2=12%) (
Figure 3).
Three studies reported the SPL during sustained phonation, reading of the Rainbow Passage, and monologue as the outcomes 6 months after treatment (
14,
15,
17). The overall effects on the SPL during sustained phonation, reading of the Rainbow Passage, and monologue were, respectively, 6.07 dB (95% CI=0.10, 12.24, p<0.00001; REM, I
2=90%) (
Figure 2), 2.89 dB (95% CI=1.27, 4.52, p<0.00001; FEM, I
2=28%) (
Figure 3), and 2.09 dB (95% CI=0.83, 3.35, p=0.001; I
2=0%) (
Figure 3) in the SLT group, compared with the control group. One study showed that only participants who underwent the LSVT had significantly increased vocal intensity during sustained vowel phonation (F=26.82, df=1, 21, p<0.0001) and reading of the Rainbow Passage (F=8.26, df=1, 21, p=0.0091) at 12 months after treatment, but no statistically significant differences were found between pretreatment and posttreatment in the placebo group (14).
STSD.
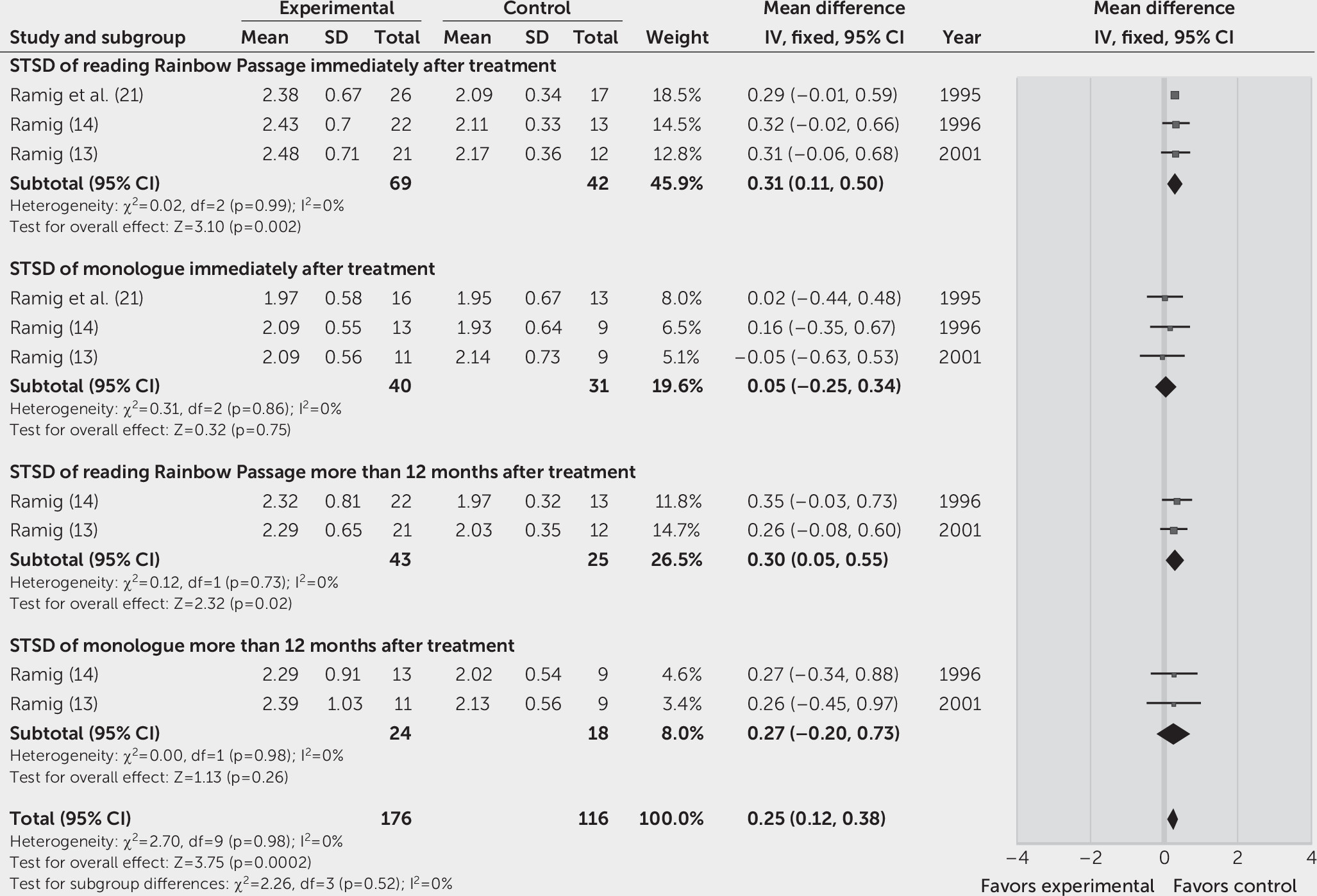
Three studies reported the STSD during reading of the Rainbow Passage and monologue as the outcome immediately after treatment (
13,
14,
21). The overall effects on reading of the Rainbow Passage and monologue were, respectively, 0.31 dB (95% CI=0.11, 0.50, p=0.002; FEM, I
2=0%) and 0.05 dB (95% CI=–0.25, 0.34, p=0.75; FEM, I
2=0%) in the SLT group, compared with the control group. Two studies (
13,
14) revealed significantly increased STSD only during reading of the Rainbow Passage (95% CI=0.05, 0.55, p=0.02; FEM, I
2=0%) but not during conversational monologue (95% CI=–0.20, 0.73, p=0.26; FEM, I
2=0%), more than 12 months after treatment in the LSVT group (
Figure 4). One study found that the LSVT significantly improved the STSD during reading of the Rainbow Passage and monologue immediately after treatment and at the 24-month follow-up, compared with pretreatment (
13).
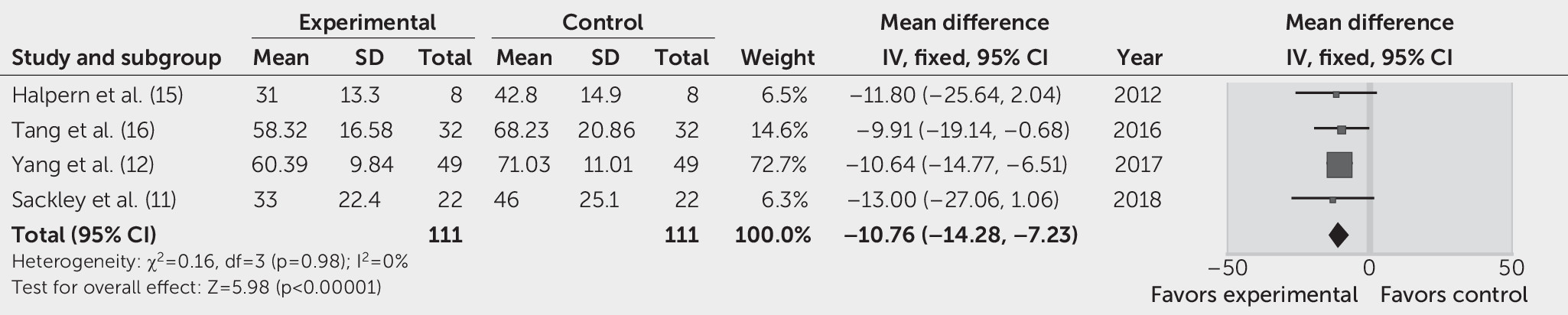
VHI.
Four studies reported VHI as the primary outcome 12 weeks after treatment (
11,
12,
15,
16). The VHI score was significantly reduced by 10.76 dB in the SLT group, compared with the control group (95% CI=–14.28, –7.23, p<0.00001; FEM, I
2=0%) (
Figure 5).
Sensitivity Analysis
Considerable heterogeneity (I
2=54%) was observed in the SPL of sustained phonation immediately after treatment in the SLT group. The leave-one-out sensitivity analysis revealed that the I
2 was materially altered (from 54% to 0%) and the SPL increased by 13.44 dB (95% CI=12.06, 4.83, p<0.00001) when one study (
9) was excluded, indicating that the heterogeneity was attributable to this study (
9). The baseline data, interventions, and the collection and evaluation of outcomes in this study (
9) were not considerably different from those in other studies in this meta-analysis; therefore, the heterogeneity may be derived from systematic errors. In addition, the heterogeneity (I
2=90%) in the SPL of sustained phonation 6 months after treatment was attributed to another study (
15), in which the SPL was lower in the SLT group than in the control group.
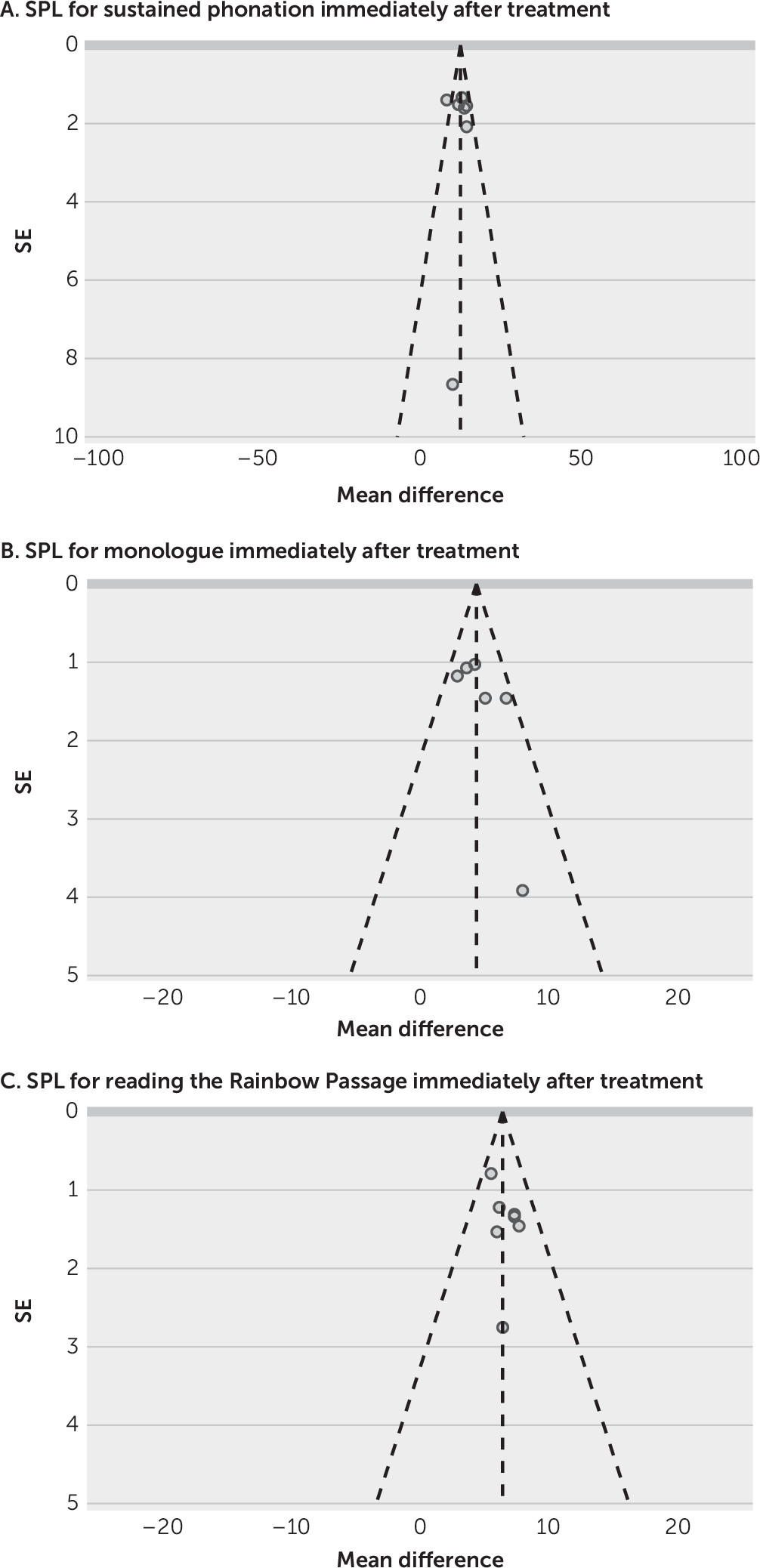
Publication Bias
No publication bias was detected in the funnel plots, which included more than five studies (
9–
10,
13–
15,
17,
21) (
Figure 6).
Discussion
This meta-analysis was aimed to evaluate the effects of SLTs on patients with PD with voice and language problems. We found that SLTs increased the SPL during sustained phonation, reading of the Rainbow Passage, and monologue 6 months after treatment, elevated the STSD during reading of the Rainbow Passage more than 12 months after treatment, and reduced VHI scores among patients with PD with dysphonia problems at least 3 months after treatment.
Many patients with PD have voice disorders for which pharmacological and neurosurgical treatments are not effective. It is well known that PD is associated with alterations in basal ganglia circuitry because of a decrease in dopamine; however, dysfunction in this circuitry may offer only a partial explanation for voice disorders in individuals with PD. Sensory deficits and decreased striato-prefrontal connectivity in internal monitoring and maintenance of amplitude of movements across the speech production mechanism may also contribute to decreased loudness, imprecise articulation, and monotone (
24–
28). These factors may also explain why the symptoms of dysphonia are not sensitive to dopamine treatment.
Prosodic exercises, one of the SLTs, decrease the perceived prosodic abnormality of speech, and intonation exercises improve speech ability. LSVT, one type of SLT, was developed in the mid-1990s by Ramig et al. (
17). This treatment focuses primarily on a simple set of tasks designed to improve maximally phonatory and respiratory functions, including respiratory drive, vocal fold adduction, laryngeal muscle activity and synergy, laryngeal and supralaryngeal articulatory movements, and vocal tract configuration (
13). LSVT differs from traditional PD voice treatments in the following ways: the singular target of treatment is voice, treatment is intensive, and the sensory component of the voice disorder is addressed (
9). Two neuroimaging studies indicate brain functional changes after classic therapy, supporting the beneficial effects of LSVT (
29,
30).
Although SLT is an effective intervention for patients with PD, it is difficult to provide because of the disparity between the number of service providers and the number of patients needing this treatment, funding issues that limit access to treatment, and geographic constraints that create barriers to care access (
31). Development of telehealth technologies may overcome some of these accessibility challenges. For example, the effect of Web-based LSVT on voice loudness is similar to that of classic LSVT but can be provided in a more time- and cost-efficient manner, compared with traditional in-person LSVT (
20). Delivering LSVT through an interactive, customized, personal digital assistant–based software program (e.g., LSVT Companion) allows delivery of this treatment despite mobility and geographical constraints, with use of LSVT-certified clinicians at a reasonable cost (
15).
This study had several limitations. The quality of the included RCTs was unclear. Some of these RCTs did not provide a detailed description of the randomization method, concealment of allocation, and blinding of outcome assessment, which rendered the risk of bias unclear. The long-term effects (1 or 2 years) on SPL, STSD of monologue, and VHI were not identified. The severity of voice disorders in most participants was classified as mild to moderate, and the efficacy of SLTs was not assessed in patients with PD with severe conditions. Information on patient adherence or compliance to an intensive therapy program and information about the maintenance plan was unavailable in the included studies. It should be noted that statistical significance does not mean clinical significance. Some of the reported statistically significant differences in decibel levels in the meta-analysis were around only ≤3 dB—generally below a detectable change for human perception.
Conclusions
This meta-analysis demonstrated the efficacy of SLTs, especially LSVT, in increasing vocal loudness and functional communication among individuals with PD. Further RCTs with large sample sizes and multicenter participation are needed to validate the long-term effects and efficacy of SLTs in patients with severe PD.
Acknowledgments
The authors thank Editage personnel (
www.editage.cn) for English-language editing.