The pathophysiologic basis of involuntary motor symptoms in functional movement disorders (FMDs) remains poorly understood. There is emerging evidence from recent neuroimaging studies linking FMD to aberrant activity and morphology in prefrontal and basal ganglia circuitries (
1–
7). While these cortical-striatal abnormalities may be connected to the expression of aberrant movements, such as tremor and dystonia, dysfunction of prefrontal-basal ganglia circuitries is also responsible for deficits in the cognitive control of movement. Specifically, a network connecting the dorsomedial prefrontal cortex, inferior frontal cortex, presupplementary motor area, striatum, and subthalamic nuclei is implicated directly in the inhibitory control of movement (for a review, see Aron et al [
8]), which appears to be an intriguing system to investigate in FMD. It is noteworthy that Aron et al. (
8) associated both stopping control and conflict control with frontal-striatal circuits. The aim of the present study was to investigate both types of control in patients with FMD.
A go-no-go task was used in two previous investigations reporting that individuals with FMD committed higher rates of errors on no-go trials (i.e., they reacted when they should have withheld a response), which suggests that FMD interferes with inhibitory control in situations with a strong response bias (
5,
9).
In the present study, we investigated whether FMD exerts a dissociable effect on two forms of inhibitory control: the latency to stop an intentionally initiated action (action cancelation) and the proficiency in inhibiting unintentional action impulses (interference control).
To test this, participants performed three well-established cognitive tasks: a choice reaction task, a stop-signal task, and a Simon task. A basic two-choice reaction task quantified action initiation speed. The stop-signal task also provides an estimate of action initiation speed and a measure of action cancelation speed (stop-signal reaction time). Slower stopping latencies reflect increased difficulty in inhibiting ongoing intentional actions (
10). Finally, the Simon task provides measures of conflict between goal-directed actions and automatic (unintentional) action impulses (
11). The Simon task calls for a left or right response to a particular feature of a spatially lateralized stimulus (i.e., arrow direction). Reduced interference control is reflected by slower and less accurate responses when stimulus location and the action signaled by the arrow direction do not correspond (i.e., right hand response to a right-pointing arrow in the left visual half-field), compared with when they correspond.
We hypothesized that the dysfunction of prefrontal-basal ganglia circuits in patients with FMD would interfere with inhibitory motor control. Our first prediction was that the latency to inhibit an intentionally initiated movement would be slower among patients with FMD (slower action cancelation in the stop-signal task) compared with healthy control subjects. The demonstration that patients with FMD are slower to stop intentionally initiated movements could have interesting implications clinically. Second, we predicted that the inhibitory dysfunction in patients with FMD might be related to disruption in the ability to inhibit unintentionally triggered action impulses. This would imply that patients with FMD have difficulty inhibiting unintentionally activated movements (reduced interference control as measured by the Simon task).
Methods
Participants
Patients with FMD (N=30) were diagnosed using criteria by Williams et al. (
12). Diagnosis was made by a board-certified neurologist (K.L.), and participants were recruited from the Movement Disorders Clinic at the University of Louisville Physicians group. Motor severity was evaluated in FMD patients using the Simplified Functional Movement Disorders Rating Scale (S-FMDRS) (
13). Healthy control subjects (N=53) were recruited via community advertisement at the Vanderbilt University Movement Disorders Clinic (21 healthy control subjects performed the Simon task, and another 32 performed the stop and choice reaction tasks). Demographic and clinical characteristics of the study participants are summarized in
Table 1.
Individuals with medical, neurological, or psychiatric comorbidities known to interfere with cognition were excluded on the basis of a standardized health eligibility questionnaire. A Montreal Cognitive Assessment score of 22 was used as the lower limit of cognitive performance for inclusion in the study.
Participants provided informed consent before participation in any study procedures, in full compliance with the standards of ethical conduct in human investigation as regulated by the institutional review boards of Vanderbilt University and the University of Louisville.
Experimental Tasks and Procedures
Participants completed a choice-reaction task, a stop-signal task, and a Simon task, measuring action initiation, action cancelation, and interference control, respectively. A detailed description of each task is provided in the
online supplement. All study subjects completed the choice-reaction task first and subsequently performed the stop-signal and Simon tasks. Patients with FMD also completed three questionnaires to rate the severity of somatic symptoms: the Patient Health Questionnaire-15 (PHQ-15) (
14), the Generalized Anxiety Disorder-7 (GAD-7) scale (
15), and the Patient Health Questionnaire-9 (PHQ-9) (depressive symptoms) (
16).
Data Analyses
Choice-reaction task.
We compared go reaction times and accuracy rates from the choice-reaction task between the study groups.
Stop-signal task.
Analyses of variance (ANOVAs) were performed in order to compare go reaction times and stop-signal reaction times from the stop-signal task as a function of group (FMD and healthy control subjects) to test whether action initiation and action cancelation were reduced in patients with FMD compared with control subjects. Stop-signal reaction times were estimated by using the horse-race model and integration method (
17). Participants whose performance violated the horse-race model (<35% stop success, signal response reaction time larger than the mean go reaction time) were excluded from stop-task analysis (N=3).
Simon task.
Reaction time latencies for corresponding and noncorresponding trials faster than 150 ms (anticipatory reactions) were excluded. For each level of correspondence (i.e., corresponding and noncorresponding), mean reaction times and log-transformed accuracy rates were calculated to approximate normal distribution. Participants with >50% omission or commission errors on corresponding trials were excluded from the Simon analysis (N=4).
Similar to our previous work using the Simon task (
18,
19), we used the dual process activation suppression (DPAS) model to dissociate fast response impulses and inhibition of incorrect responses. Distributional analyses were implemented by rank-ordering single-trial reaction times from fastest to slowest and by dividing reaction times into six equal-sized bins.
According to the DPAS model, impulse capture is measured by plotting the accuracy rates against the reaction time for each level of correspondence (conditional accuracy function). The proportion of fast errors on noncorresponding trials (i.e., within fastest reaction time bin) reflects the strength of the incorrect response capture (
20).
The DPAS also allows measurement of the proficiency of inhibitory control over time by means of delta plots, which show the size of the Simon effect on reaction time as a function of the reaction time. According to the DPAS model, when an incorrect response has been triggered by noncorresponding stimulus information, it takes time for inhibitory control to build up. Therefore, the proficiency of inhibitory control is most clearly reflected at the slope of the interference reduction between the final two (slowest) reaction time bins of the delta plot (delta slope) (
21). A flat or positive-going delta slope indicates reduced interference control, which would be predicted for patients with FMD compared with control subjects.
We first compared reaction times and accuracy rates from the Simon task by means of an ANOVA, within-subjects factor correspondence (corresponding, noncorresponding), and between-subjects factor group (FMD patients, healthy control subjects). Second, we performed ANOVAs to compare final delta slopes and fast impulsive errors as a function of group.
Correlations Between Cognitive Performance and Clinical Measures
We also correlated ratings of patients’ clinical motor symptom severity (measured with the S-FMDRS), depression, anxiety, and somatic severity with measures of action control (go reaction time, stop-signal reaction times, delta slopes, and impulsive errors).
Results
Demographic characteristics and clinical variables of the study participants, as well as group effects on mean reaction times and accuracy rates of the action-control tasks, are summarized in
Table 1.
Participants’ Demographic Characteristics
Predominant symptoms of patients with FMD were present with the following frequencies: tremor (53.3%), dystonia (20%), gait disorder (10%), myoclonus (10%), and tics (6.7%).
Healthy control subjects who performed the choice-reaction and stop-signal tasks were similar in education level (p>0.1) but older in age (F=36.3, df=1, 58, p<0.05) and more likely to be male (χ2=10.0, df=1, p<0.05) compared with patients in the FMD group. Healthy control subjects who performed the Simon task were similar in age and sex distribution (all p values >0.2) but had more years of education compared with patients in the FMD group (F=11.2, df=1, 45, p<0.05).
Choice-Reaction Time Task (Action Initiation)
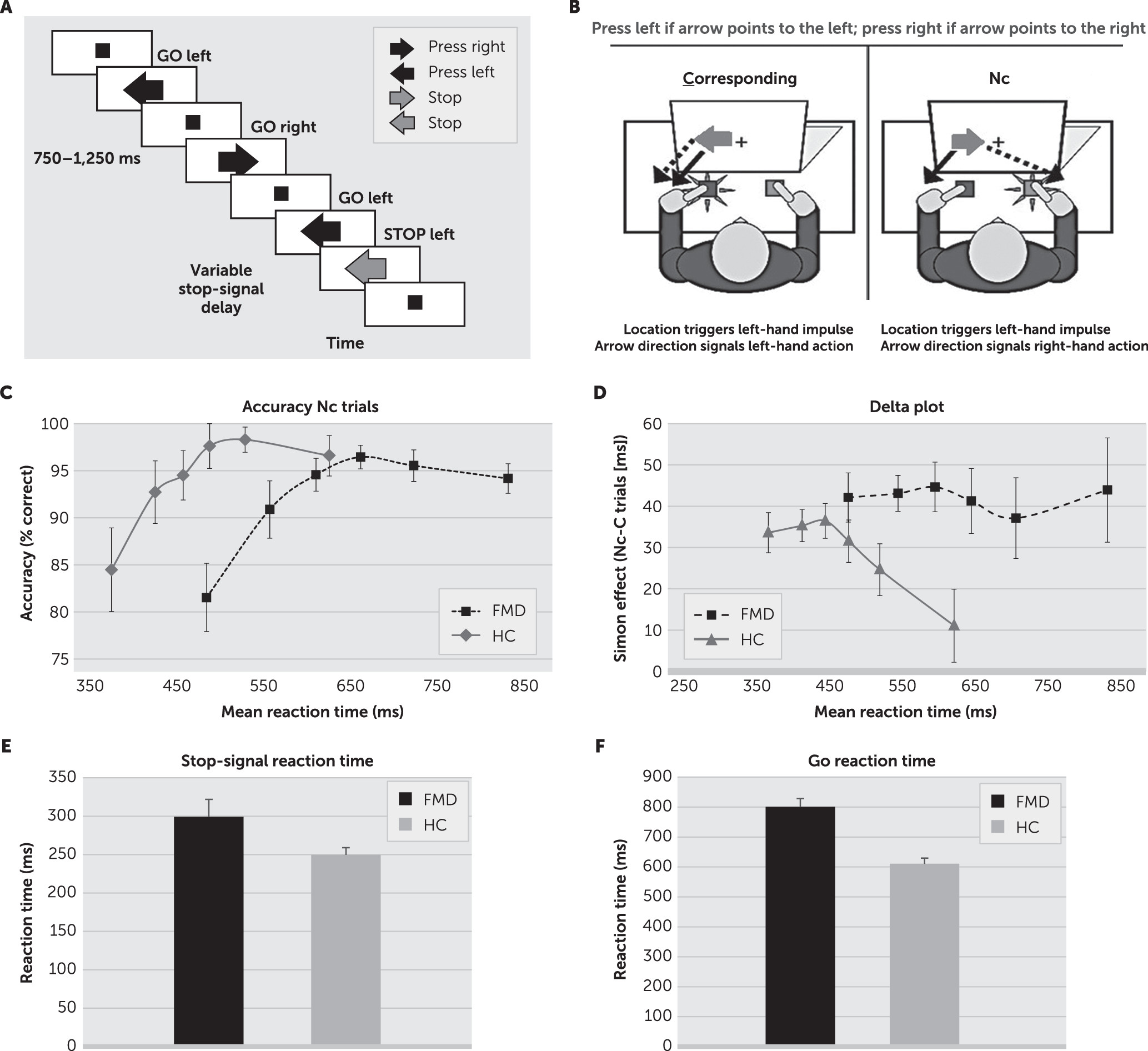
Patients with FMD were significantly slower to initiate actions than healthy control subjects, as evidenced by longer go reaction times on the choice-reaction time task and the stop-signal task (choice-reaction time task: F=11.79, df=1, 57, p<0.01, mFMD=575 ms, mHC=472 ms).
Stop-Signal Task (Action Cancelation)
Patients with FMD were significantly slower to initiate actions than healthy control subjects, as evidenced by longer go reaction times on the stop-signal task (F
stop=35.16, df=1, 58, p<0.001,
mFMD=801 ms,
mHC=611 ms). Patients with FMD were also slower to stop voluntary actions (slower action cancelation), expressed by longer stop-signal reaction times (stop-signal reaction times; F=4.6, df=1, 58, p<0.05
mFMD=299 ms,
mHC=250 ms) (
Figure 1). Go reaction times and stop-signal reaction times were independent in both groups (i.e., go reaction times and stop-signal reaction times did not correlate in either group, r
FMD=0.17, p=0.4; r
HC=0.17, p=0.35), indicating independence of action initiation and action cancelation.
Simon Task (Interference Control)
Performance on noncorresponding (Nc) trials was slower and less accurate than on corresponding trials (reaction time: F=75.69, df=1, 45, p<0.001; accuracy: F=20.26, df=1, 45, p<0.001), but there was no interaction with group (F values <2.6, df=1, 45, p values >0.12). Across Simon conditions, patients with FMD were slower to respond than control subjects but equally accurate (reaction time: F=28.67, df=1, 45, p<0.001; accuracy: F<1.0, df=1, 45, p >0.9).
Moreover, the delta-slope analysis demonstrated that the proficiency of inhibitory control was significantly impaired in the FMD group
(F=4.56, df=1, 45, p<0.05) (
Figure 1). That is, the inhibition slope (i.e., the delta slope between the last two bins of the reaction time distribution) in patients with FMD (
m=0.05) was positive going compared with the inhibition slope among healthy control subjects (
m=–0.13), which reflects poor inhibition of involuntary action impulses (poor interference control) among patients with FMD. Response impulse-capture rates (accuracy rates in the fastest reaction time bin of the Nc trials), on the other hand, were similar between the two study groups (F=0.02, df=1, 45, p=0.87) (
Figure 1).
Correlations Between Behavior and Clinical Measures
Increased somatic severity on the PHQ-15 was related to slower stopping reaction times (p=0.01, r=0.51); however, this finding did not hold for Bonferroni correction. No other significant correlations were found between clinical and action-control variables (r values <0.39, p values >0.12).
Discussion
Individuals with FMD present with aberrant movements, such as tremor and dystonia. There is considerable debate regarding the origins of these movements, including whether they are intentional or unintentional. We investigated how FMD affects the ability to inhibit intentionally initiated movements (action cancelation as measured by the stop-signal task) and the ability to inhibit unintentionally (automatically) activated motor impulses (interference control as measured by the Simon task), which represent dissociable inhibitory control systems. Participants with FMD were globally slower to react than healthy control subjects. Independent of this motor slowing, FMD was associated with slower stopping latencies on the stop-signal task when trying to inhibit an intentionally initiated action, as well as with less proficient inhibition of unintentionally triggered motor impulses, as evidenced by the delta slope in the Simon task. Thus, FMD appears to have a global effect on forms of inhibitory motor control, irrespective of the putative sources of the involuntary movement disorder associated with the condition. The presence of inhibitory control dysfunction is consistent with imaging studies showing altered frontal-basal ganglia circuitry in FMD (
1–
6), although future studies need to establish a direct link.
Limitations
There are several limitations to this study. The healthy control subjects who performed the stop-signal task were not age matched with the patients in the FMD group. However, the significant impairment in stopping speed observed in the FMD group compared with that found among older healthy control subjects would likely be even more pronounced in contrast to a younger control group. Another limitation is that patients in the FMD group were tested while they were taking their regular medications (e.g., antidepressants, analgesics, muscle relaxants), and thus the impact of these drugs on performance could not be quantified. Moreover, healthy control subjects were not tested regarding anxiety and depression, and therefore the results of the FMD group can exclusively be contrasted with a normative group only. Anxiety and depression scores were respectively mild and moderate and did not correlate with any of the cognitive measures. However, task errors could have been processed differentially in the FMD group as a result of anxiety or mood problems, which could influence action control on a trial-by-trial basis. Future studies should explore the influences of mood and anxiety on action control in a larger sample.
Conclusions
This study suggests that patients with FMD are significantly delayed in their latencies to stop intentionally initiated movements and in their proficiency to inhibit unintentional motor impulses. Clinically, an improved understanding of how FMD affects action-control processes might advance evaluation techniques and diagnostic criteria and pave the way for the development of more effective therapies.