Brain imaging techniques are currently available for the assessment of many of the branches of pathological cascade, including vascular insufficiency (cerebral blood flow imaging [15O]water], arterial spin labeling), glucose metabolic disturbances ([18F]fluorodeoxyglucose [FDG]), β-amyloid clearance and plaques (amyloid imaging agents, e.g., [18F]florbetapir, [18F]florbetaben, [18F]flutemetamol, and [11C]Pittsburgh Compound B [PiB], tau pathology (e.g., [18F]flortaucipir), and cell death with associated atrophy (structural MRI). However, each technique examines a specific component of AD-associated pathology in isolation. None of these techniques supplies a composite picture of the status of the individual on the dementia pathological trajectory; however, each pathology contributes to cognitive deterioration. Imaging measures that vary with the overall burden or that assess a common feature (e.g., acidosis) of pathologies that affect neuropsychological function may represent a means of compositing all of these pathological processes into a single metric.
Several previous studies demonstrated that T
1rho MRI (spin-lattice relaxation in the rotating frame) is sensitive to pH (
5–
10). A consistent finding in these studies was that higher T
1rho relaxation times corresponded to a lower pH (higher T
1rho=lower pH=more acidic). This relationship held across a wide range of magnetic field strengths (3.0–9.4 T) and spin-lock amplitudes (2.9–94 μT). In addition, Owusu et al. (
11) found that the strongest contribution to changes appears to be pH at 3-T using spin-lock amplitudes that can be achieved with U.S. Food and Drug Administration limits of subject heating. Other studies (
7,
12–
14) have evaluated the use of T
1rho MRI to examine brain pH, and some have explored the potential utility of this technique in distinguishing the pathologies underlying panic disorder (
15), bipolar disorder (
16,
17), and premanifest Huntington’s disease (
18). In each disorder, the T
1rho signal was elevated in specific regions known to be adversely affected in patients with the particular neuropsychiatric disorder compared with control subjects (e.g., the striatum in Huntington’s disease [
18]).
The purpose of the present study was to investigate the relationship between T
1rho imaging and AD-associated pathologies as determined by available diagnostic imaging techniques. The techniques applied utilized measures of brain metabolism, specifically glucose metabolism with FDG, and AD-associated pathology, specifically amyloid burden with [
11C]PiB positron emission tomography (PET) imaging. It was hypothesized that higher T
1rho signal would be associated with more abnormal function or greater pathological burden, specifically lower FDG uptake, greater AD-associated pathology (i.e., amyloid burden), and poorer cognitive performance. Age was considered as a potentially important covariate in the analyses because of the conflicting findings regarding the association between T
1rho and age. Zhao et al. (
26) observed significant increases in T
1rho signal associated with age-related changes in rat brain. These findings are consistent with those of Menyhárt et al. (
27), who found tissue acidosis in aged rat brain due to alterations in pH shift induced by spreading depolarization. It was hypothesized that the spreading depolarization-induced acidic pH shift was the result of a persistent elevation in the lactate concentration due to reductions in the facilitated diffusion of lactate into the bloodstream in aged brain.
In human studies, some authors have found no relationship between T
1rho and age (
21–
23) or nonstatistically significant increases in the T
1rho signal and age (
20) when examining limited areas of the brain (e.g., the medial temporal lobe and hippocampus). However, Watts et al. (
28) found significant decreases with age in cortical gray matter and in the caudate, putamen, hippocampus, amygdala, and nucleus accumbens but significant increases with age in white matter tracts. Recent in vitro studies of human neocortical tissues resected from patients who had epilepsy surgery revealed a mild acidification with age (
29). It was hypothesized that the age-related decrease in neuronal intracellular pH may have been associated with beneficial neuroprotection by limiting excitotoxicity. On the other hand, intracellular acidification can induce apoptosis and promote the development of Alzheimer’s dementia (
30). Therefore, age was considered in all analyses.
Discussion
Currently, there are no disease-modifying therapies available for AD. Increasing evidence points toward the necessity of treating AD in the presymptomatic stage rather than waiting for the advent of a specific threshold initiation of a pathological cascade. However, the ability to diagnose AD depends on diagnostic tests performed in isolation, and for a given level of cognitive impairment, the biomarker profile can be markedly different between individuals (
37). An in vivo measure that characterizes the brain microenvironment or pathological burden, such as T
1rho, has the potential to provide a means to place an individual on the pathological continuum and to follow the trajectory over time better than single-function or pathology-oriented measures. Furthermore, this measure can be used serially without concern for the accumulated radiation burden associated with FDG, amyloid, and tau imaging.
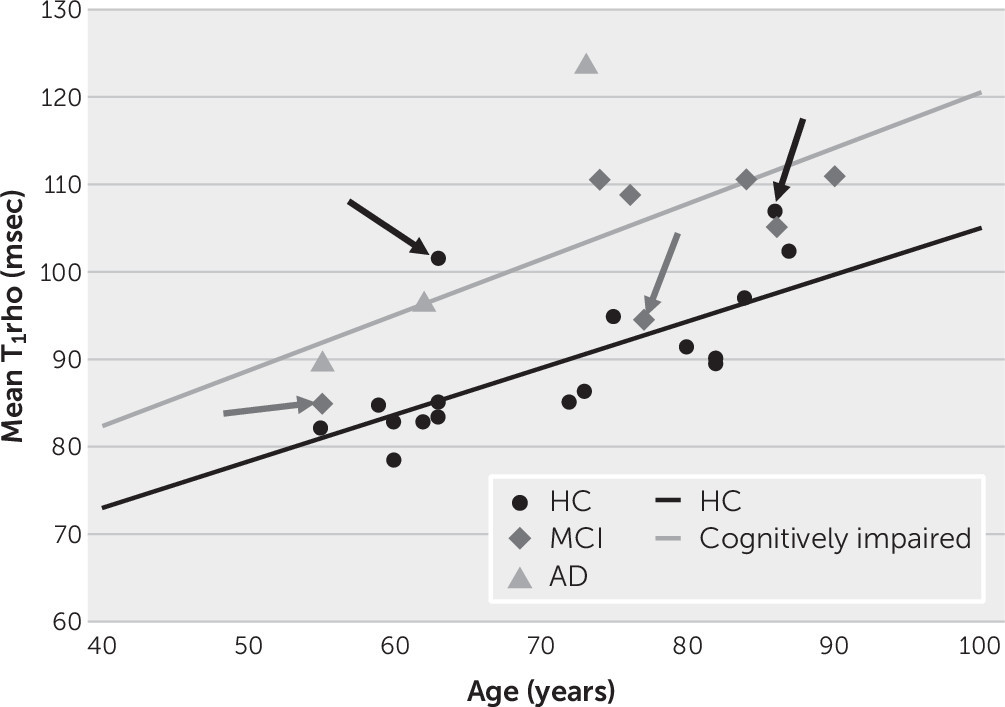
It was found that T
1rho increased with age and with increasing AD-associated pathologies. This observation is consistent with the reported relationship between age on brain pH. Bonnet et al. (
29) reported a correlation between age and pH in the middle temporal gyrus of r=−0.68. In the present study, the correlation between age and T
1rho (inversely related to pH) was r=0.57 and r=0.53 in the left and right superior posterior temporal gyrus, respectively, and 0.88 and 0.82 in the left and right posterior temporal lobe, respectively. Therefore, the relationship between age and a surrogate for pH (i.e., T
1rho) was comparable to the direct relationship observed between age and pH. Beyond this relationship and consistent with the role of acidosis in the pathological cascades, after the analysis controlled for age, T
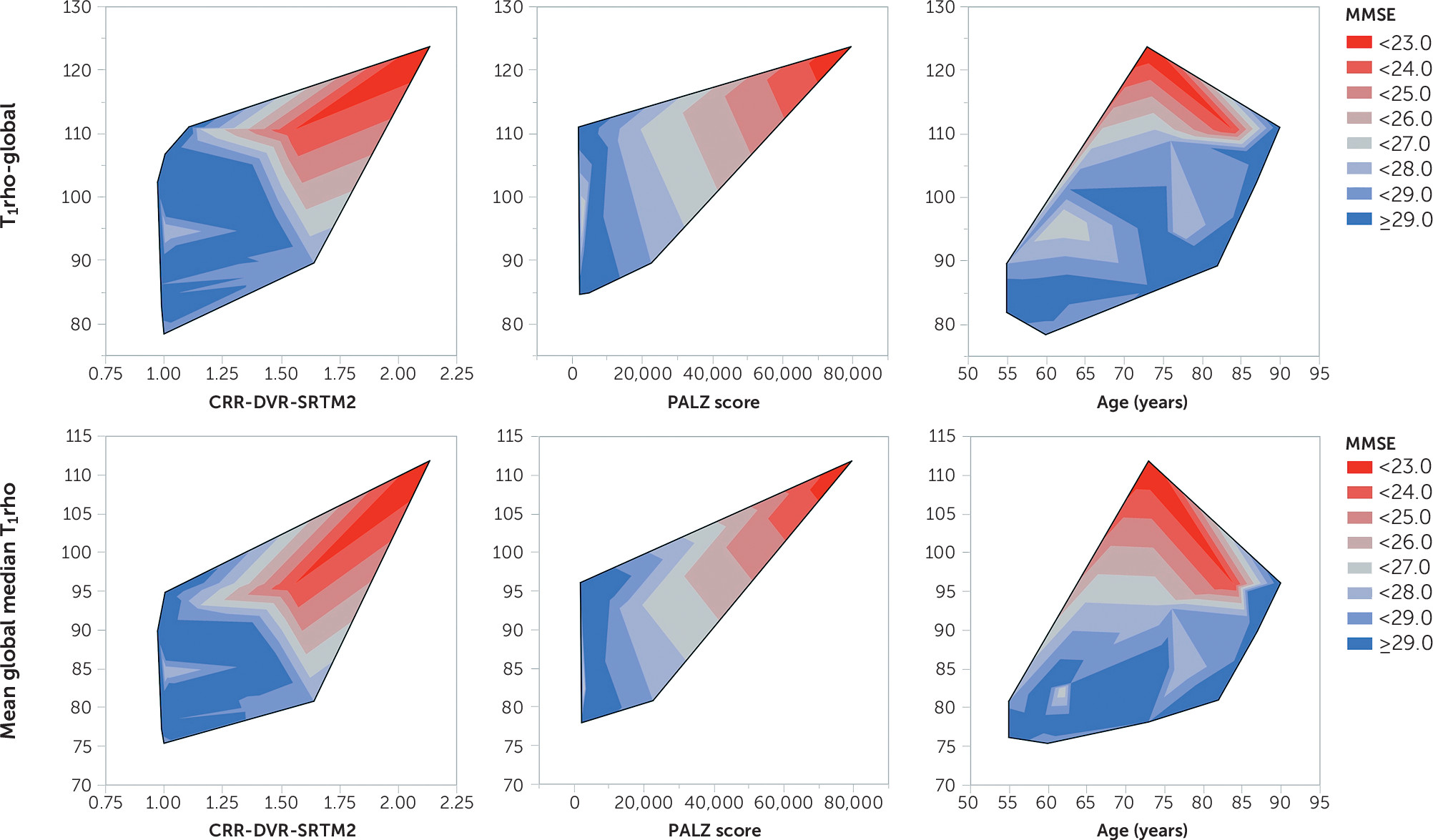
1rho increased with impaired cognition (decreasing MMSE scores and poorer immediate and long-term memory scores), increasing amyloid burden (based on PiB-based cortical retention ratios), and more aberrant glucose metabolism (based on FDG-based metabolism).
Previous work exploring the potential utility of T
1rho imaging in AD has exhibited promising results but has been hampered by limitations regarding the extent of brain characterized (e.g., medial temporal lobe only) and the classification of study subjects and pathologies (e.g., clinical diagnoses only). Borthakur et al. (
20) examined the T
1rho signal in medial temporal lobe gray and white matter in participants with AD, compared with those with MCI and with HCs. On average, participants with AD had a 6% increase in T
1rho, compared with HCs (8% for white matter and 5% for gray matter), with participants with MCI having an intermediate mean value. The HC and MCI groups tended to show a somewhat bimodal distribution in white matter T
1rho values, assumed to be due to existing premorbid pathology in some study subjects in these groups. Participant classification was based on neuropsychological tests, not knowledge of AD-specific pathologies. Although positive change in T
1rho in white matter and gray matter was exhibited with age, neither relationship was statistically significant. In our study, in which age was found to be a statistically significant factor, a 70-year-old individual with cognitive impairment would exhibit global mean and median T
1rho values that were 7.2% and 4.9% higher, respectively, compared with their HC counterparts, values consistent with the differences reported in the literature.
Gray matter and white matter T
1rho in the medial temporal lobe in participants with AD or with MCI and in an age-matched control group were examined by Haris et al. (
22). T
1rho values in gray matter and white matter, respectively, were lowest in the HC group (87.5 ms [SD=1.2] and 80.5 ms [SD=1.4]), intermediate in the MCI group (90.9 ms [SD=1.3] and 84.1 ms [SD=2.7]), and highest in the AD group (91.9 ms [SD=0.8] and 88.3 ms [SD=1.3]). These differences were statistically significant between participants with AD and HCs but not between participants in the MCI and AD groups or between participants in the MCI and HC groups. No significant relationships between age and T
1rho and between MMSE scores and T
1rho were observed. Five of the HCs had significantly increased T
1rho in both gray and white matter.
Haris et al. (
21) also examined the hippocampal (right and left) T
1rho in control subjects and in patients with AD, PD, or PDD. T
1rho was significantly increased in patients with AD compared with both HCs and patients with PD but was not significantly different from patients with PDD. T
1rho was decreased in patients with PD compared with control subjects and patients with PDD. In contrast to the present study, no significant correlations were observed between T
1rho and age or T
1rho and MMSE scores when the analysis was limited to the hippocampal areas only. However, the authors concluded that serial T
1rho imaging may provide insight into AD and PD disease progression and may assist in the early diagnosis of these diseases.
In addition, Haris et al. (
23) evaluated the sensitivity and specificity of the combination of T
1rho of the white matter and gray matter in the medial temporal lobe in combination with CSF biomarkers (β-amyloid
1–42; T-tau and P-tau, 181 p levels) for categorizing individuals with AD or with MCI and in control subjects who were classified on the basis of a clinical consensus diagnoses. Using binary logistic regression, the combination of T
1rho and CSF biomarkers predicted 86.4% of control subjects and 66.7% of participants with MCI for the comparison between the control and MCI groups and 59.3% of MCI participants and 84.6% of AD participants for the comparison between the MCI and AD groups. Receiver-operating characteristic analyses revealed that T
1rho had greater sensitivity (HC group versus MCI group, 0.60 versus 0.53; HC group versus AD group, 0.82 versus 0.77, for T
1rho and CSF biomarkers, respectively) and CSF biomarkers had greater specificity (HC group versus MCI group, 0.77 versus 0.82; HC group versus AD group, 0.71 versus 0.79, for T
1rho and CSF biomarkers, respectively) in distinguishing participants with MCI or AD from HCs. The investigators did not find any significant correlations between T
1rho and CSF biomarkers or between T
1rho and age. They concluded that the combination of T
1rho and CSF biomarkers showed promise as a specific and early diagnostic measure for AD and may easily track the progression from MCI to AD.
In line with the literature, significant differences were found between the clinical diagnostic classifications and T1rho values (HCs lower than participants with cognitive impairment); however, by expanding the T1rho field of view beyond the hippocampus and medial temporal lobe, the relationships between global T1rho and age and global T1rho and MMSE scores were discernible. Follow-up is needed to discern whether these differences translate to altered risk of deterioration. Analyses of regional T1rho values revealed significant relationships only marginally with the medial temporal structures but primarily with frontal, parietal, and cingulate structures, areas known to have AD-associated pathology (glucose hypometabolism and amyloid deposition).
T
1rho MRI is a pH-sensitive measure, with higher values consistent with a more acidic environment. The overall consistency of higher T
1rho values with greater cognitive impairment and pathological burden is concordant with the development of acidosis as an integral component of the various AD-associated pathological cascades as described by Humpel (
1). Having a marker that varies according to a feature common to each of the cascades rather than one unique only to a particular pathology presents the possibility of placing an individual on a pathological trajectory rather than exhibiting a constellation of biomarker values for a particular level of impairment (
37). T
1rho MRI holds promise as a potentially useful biomarker providing unique information beyond the T
1-weighted measures of neurodegeneration.
However, our study has several limitations that warrant further investigation before the full utility of T
1rho MRI can be determined. First, it should be noted that T
1rho imaging is not exclusively sensitive to pH, and other factors, such as glucose (
38) and glutamate (
5) concentrations, have been shown to influence T
1rho relaxation times at higher magnetic fields and spin-lock amplitudes. In AD, there are known changes in protein aggregates and in glutamate and glucose concentrations that could influence T
1rho relaxation times. Thus, it cannot be ruled out that other metabolites may be driving the T
1rho relaxation time changes observed in this study. However, recent phantom work conducted by Owusu et al. (
11) revealed that the T
1rho signal was more sensitive to changes in physiologic pH than to changes in glucose or lysine concentrations in a protein-rich medium (egg-white albumin phantom) at physiologic temperatures, adding assurance that the observed in vivo T
1rho signal likely reflects pH. Second, it is currently unknown whether there are dispersion effects of the spin-lock pulse in vivo that would vary its sensitivity across regions of the brain. Future studies might utilize several spin-lock amplitudes to evaluate dispersion effects and regional sensitivity. Third, our study sample was small and skewed toward HCs, with participants with AD limited to the lower half of the age range only. A broader range of older participants with more extensive pathology will be needed to fully explore the interactions between age, cognitive impairment, and pathological burden.
Fourth, and most important, this study was cross-sectional only. In order for T1rho MRI to be an effective metric of AD-associated pathology, it needs to be reproducible (i.e., produce the same values on day 1 compared with day 2), reliable (i.e., exhibit the same relationship between the T1rho measure, cognition, and other pathological measures at time 1 and time 2), and longitudinally consistent (i.e., on an individual basis, increasing T1rho would equal decreasing cognitive performance; stable T1rho would equal stable cognition). Reproducibility data were not available for our study population and scanner configuration. However, recent investigations conducted at this institution (data available upon request from Dr. Laura L. Boles Ponto) using a state-of-the-art, high-resolution, efficient sagittal segmented three-dimensional gradient echo sequence (TE=2.5 ms, TR=5.6 ms, field of view=220×220×160 mm3, matrix=128×128×80, flip angle=10°, and acceleration=2, spin-lock amplitude=400 Hz, spin-lock durations=0 and 80 ms) implemented on a 3.0-T GE Discovery 750W MRI scanner revealed that for a limited group of study subjects (N=5), T1rho values were highly reproducible in the short-term (i.e., 7–14 days) both regionally and globally. For a combination of subcortical and cortical hemispheric regions (N=20 per subject), the correlations between time 1 and time 2 were 0.90 and 0.92, respectively, with average differences of –1.7% and –1.1% for mean and median values, respectively. Data are currently not available to address the reliability and longitudinal consistency of these measures but are the basis of future T1rho-related research at this institution.