Vascular dementia is recognized as one of the most common causes of major cognitive impairment after Alzheimer’s disease (
1–
3). Currently, the lesional markers of vascular dementia and their relative contributions are better known. Several brain MRI markers, such as stroke subtype (ischemic or hemorrhagic), stroke volume and gross location (
4,
5), recurrent strokes (
2,
6), the presence and extent of white matter hyperintensities (
7,
8), brain microbleeds (
9,
10), and medial temporal lobar atrophy (
11), may produce complex neurocognitive symptoms and dementia. Multiple brain infarcts have also been suggested to be an important risk factor for dementia.
The term “multiple brain infarcts” has been used to define a variety of multiple ischemic lesions that appear in one or both hemispheres or in the anterior and posterior circulation territories (multilevel) (
12). Multiple ischemic lesions in different parts of the brain may present specific clinical patterns as described in previous studies (
13,
14). A large body of evidence suggests that simultaneous multiple cortical and subcortical ischemic lesions in the anterior and posterior circulation are linked to the incidence of cognitive decline among healthy people (
15,
16). Despite these new insights, to our knowledge, there are no systematic studies of stroke cohorts investigating associations between simultaneous multiple infarcts and cognitive decline among dementia-free individuals.
In this study, we aimed to clarify the risk of dementia after first stroke in the anterior and posterior circulation territories in a large, prospective stroke cohort.
Methods
Between September 2004 and December 2017, 11,200 patients with ischemic stroke were prospectively included from the Ege University Medical Center. Details of the study cohort have been described previously (
17). All patients who were hospitalized (<30 days) for ischemic stroke, with initial positive imaging, a reliable informant from among caregivers or family members, and no previously diagnosed disorders affecting cognition, were included. Clinical, neuropsychological, and MRI examinations were performed during the first month of participant enrollment in the study. Baseline examinations and definitions of vascular disease (
18) and risk factors are described briefly below. The prior cognitive status of each patient was requested from his or her primary care physician or from Turkey’s National Health Data system.
Prospectively recorded variables included age, gender, risk factors, blood pressure, ischemic stroke characteristics, location, pathogenesis, disability (determined using the National Institutes of Health (NIH) Stroke Scale [
19] and the modified Rankin Scale [
20]). clinical subtypes, topography of infarcts on diffusion-weighted imaging or fluid attenuation inversion recovery (FLAIR) MRI studies, and neurologic and systemic complications. The combination of computerized tomography (CT) scan and angiography was the routine of our stroke unit for all patients with stroke. MRI was performed within 24 hours of admission by using 1.5- and 3-T scanners (Siemens Sonata, Siemens Medical Solutions, Erlangen, Germany). To differentiate acute from chronic lesions, T
1 and T
2, diffusion-weighted imaging and FLAIR MRI sequences were used concomitantly. The diffusion-weighted imaging sequence was obtained using echoplanar imaging (field of view=230 mm; slice thickness=5 mm; repetition time [TR] 3,200 ms; echo time [TE]=94 ms). All CT and MRI scans were reviewed by two neuroradiologists with extensive CT and MRI experience who were blind to clinical status. The kappa coefficients (interexaminer reliabilities) for lesions including infarct, leukoaraiosis, brain microbleeds, and dilated perivascular spaces were 0.98, 0.97, 0.96, and 0.98, respectively, for FLAIR and diffusion-weighted imaging.
The study was approved by the ethics committee of the Ege University Medical Center, and written informed consent was obtained from all participants.
Assessment of Poststroke Cognitive Status
The evaluation of cognitive function was performed by a neurologist (E.K.) and a clinical neuropsychologist (H.A.) 3 months after stroke, because mental status may be affected by pathophysiological factors during the acute hospitalization, and thus patients may be falsely classified as having vascular dementia. Dementia (referred to as a neurocognitive disorder diagnosis in DSM-5 after 2015) was determined in accordance with DSM-IV and DSM-5 criteria (
21). Therefore, the diagnosis of dementia or major neurocognitive disorder was made if cognitive deficits interfered with the patient’s independence in everyday activities and if significant cognitive decline from a previous level of performance in one or more cognitive domains was present. Normal cognitive status was determined using evidence of normal cognitive performance in cognitive domains without interference with the capacity for independence in everyday activities, which was measured by the Bristol Activities of Daily Living Scale (
22). Global mental function was evaluated in each patient using the Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA) batteries (
23).
Lesion Patterns
We established diagnostic MRI criteria to identify multiple cerebral infarcts in different arterial territories, defined as at least two diffusion-weighted imaging or FLAIR lesions in at least two different arterial territories (right or left anterior or posterior cerebral circulation) (
18,
19). Multiple acute infarcts in one cerebral hemisphere was considered in patients who had infarcts with combinations involving the territories of the middle cerebral artery, the anterior cerebral artery, and the anterior choroidal artery. Multiple acute bihemispheric infarcts in the anterior circulation were presumed in patients with acute multiple infarcts involving both hemispheres with lesions either in superficial or deep arterial territories. Multiple acute infarcts in the posterior circulation were determined in patients with concomitant infarcts in the territory of the vertebral arteries, the basilar artery, the superior cerebellar artery, the anterior inferior cerebellar artery, the posterior inferior cerebellar artery, and the posterior cerebral artery. Multiple acute infarcts in the anterior and posterior circulation were considered in patients with combined supratentorial and infratentorial infarcts.
The locations of strategic regions as defined previously (
3,
15,
16) were determined by two neuroradiologists with extensive CT and MRI experience who were blind to clinical status. These strategic sites included large foci (>1.5 cm) within the following regions: the middle and inferior frontal gyri, parietal region, middle temporal gyrus, the putamen and pallidum, cingulate gyrus, corpus callosum, arcuate fasciculus, middle occipital region and hippocampus, thalamus, midbrain, and postero-inferior cerebellum. The stroke lesion volume was determined using STandards for ReportIng Vascular changes on nEuroimaging criteria and a previously validated method (
16). White matter hyperintensity burden (leukoaraiosis) was assessed using the Fazekas scale score (
24) on the basis of FLAIR sequence. Brain microbleeds were defined as small (diameter, <10 mm) areas of signal void with associated blooming seen on T
2*-weighted MRI, according to the Brain Observer MicroBleed Scale criteria (
25). T
2 sequence was used to assess the presence of dilated perivascular spaces.
Stroke Mechanisms
Etiology was determined by using the Trial of Org 10172 in Acute Stroke Treatment classification (
18) and classified as large-artery atherosclerosis, cardioembolism, small-vessel disease, or other and unknown causes. Large-artery disease was presumed in patients with carotid artery stenosis >50% and diffusion-weighted imaging demonstrating multiple infarcts distal to the stenosed vessel in the territory of the relevant artery. Small-artery disease or local branch occlusion was defined in patients with infarcts <15 mm in diameter localized in the deep regions of the brain or in the brainstem without large-artery disease and cardioembolism. Cardiac embolism was determined in patients having a source of embolism. Strokes of other determined or undetermined causes were also recorded.
Concomitant Risk Factors
Hypertension, diabetes mellitus, hyperlipidemia, and current cigarette smoking, were recorded. Apolipoprotein E (ApoE) genotype was determined by polymerase chain reaction amplification of leukocyte DNA. Participants were classified by the presence versus absence of at least one ApoE ε4 allele.
Statistical Analysis
The prevalence of dementia was calculated as the ratio of the number of patients with dementia to the total number of testable patients with first-ever stroke. Proportions, odds ratios, and means of baseline characteristics were compared between patients with and without dementia using the chi-square test, logistic regression, and analysis of variance, respectively. Univariate associations among age, sex, vascular risk factors, ApoE ε4 allele, stroke subtypes, single versus multiple lesions in the anterior and posterior circulation, white matter lesions, microbleedings, and dementia were analyzed using logistic regression to estimate unadjusted odds ratios. In the second analysis, we introduced into the model all potential confounders with a probability value <0.20 in univariate analysis, and multiple logistic regression analysis by forward stepwise method (likelihood ratio) was used to find the association of dementia with single versus multiple lesions in different territories. Association between a single lesion and multiple lesions in different territories with dementia was sought to estimate models using multiple logistic regression analysis by including all lesions (model 1) and by excluding (model 2) Fazekas scale scores and microbleedings. Probability values <0.05 were considered statistically significant. All statistical analyses were performed using SPSS, version 18.0.
Results
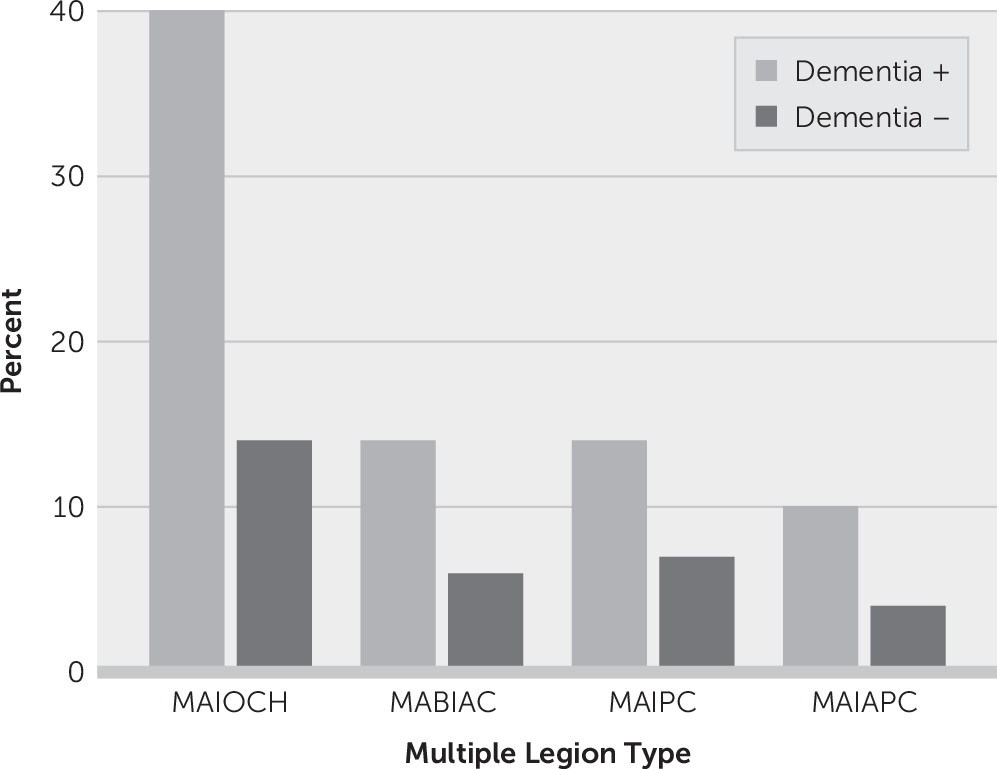
Of the 11,200 patients with ischemic stroke, 8,700 patients were eligible for neuroimaging evaluation and cognitive examination for the final analysis. Patients with old infarcts or old hemorrhagic lesions on imaging were excluded. None of the eligible patients had previously diagnosed diseases affecting cognition. Among 8,700 patients (mean age: 66 years [SD=12]; women, N=4,219 [48%]), 3,117 (36%) had multiple lesions involving anterior circulation, posterior circulation, or both on brain imaging. Vascular dementia developed in 1,048 (12%) patients; those with multiple lesions had more frequent dementia (27%) compared with patients with a single lesion (6%) (odds ratio=5.83, 95% CI=5.08, 6.70; p<0.001). Multiple acute infarcts in one cerebral hemisphere were present in 1,466 patients (17%), multiple acute bihemispheric infarcts in the anterior circulation in 612 patients (7%), multiple lesions involving posterior circulation in 663 patients (8%), and multiple lesions involving both anterior and posterior circulation in 376 patients (4%) (
Table 1,
Figure 1).
Normative cognitive data from healthy volunteers included a mean MMSE score of 27.7 (SD=1.9) and a mean MoCA score of 24.6 (SD=3.9). For patients with dementia, the mean MMSE score was 16.8 (SD=4.0), and for those without dementia, it was 26 (SD=1.9; p<0.001). For patients with multiple lesions, the mean MMSE score was significantly lower than the mean score for those with a single lesion (23.2 [SD=5.1] versus 26.1 [SD=2.7]; p=0.001).
In the univariate analysis, factors related to dementia with a p value <0.2 were age, sex, hypertension, diabetes mellitus, ApoE ε4 allele, NIH Stroke Scale score at stroke onset, causes of stroke, white matter hyperintensity burden, the presence of microbleeds, and types of multiple infarcts versus a single infarct. These factors were entered into the regression analysis (
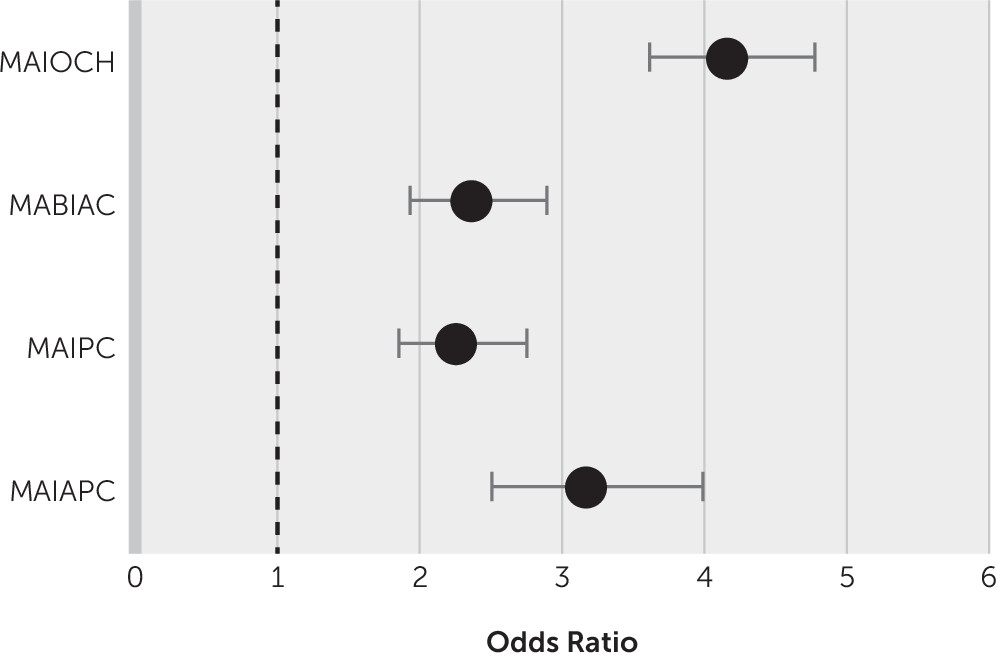
Table 2). Stepwise linear regression was used to select all types of multiple infarcts that were independently associated with dementia compared with those not associated with dementia (
Figure 2). Large-artery disease (odds ratio=1.32, 95% CI=1.01, 1.35; p=0.002) and cardioembolism (odds ratio=1.64, 95% CI=1.37, 1.96; p<0.001) were significantly associated with multiple infarcts in patients with dementia. Assessment of a possible threshold effect for leukoaraiosis burden and the presence of microbleeds did not change the final multivariate analysis findings. The ApoE ε4 allele in patients with dementia was more frequent in those with multiple lesions (17%) than in those with a single lesion (13%) (odds ratio=1.63, 95% CI=1.38, 1.93; p<0.001).
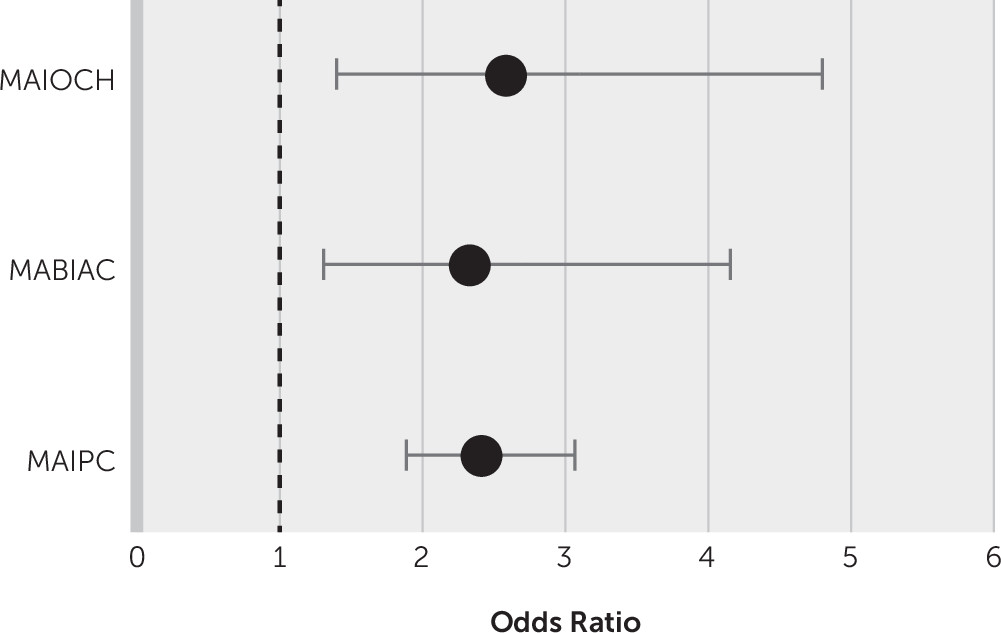
Multiple regression analysis for different location type after the exclusion of confounder factors, such as brain microbleeds and white matter hyperintensity burden, showed that multiple lesions in one hemisphere versus a single lesion (p<0.001), strategic region involvement (p<0.001), and stroke lesion volume (p=0.03) were significantly associated with dementia (
Table 3,
Figure 3). Left hemispheric lesions were significantly associated with dementia (odds ratio=2.56, 95% CI=2.11, 3.11; p<0.001).
Discussion
The clinical syndrome of vascular dementia developed in 12% of patients in this study cohort, whereas dementia was four times more frequent in patients with multiple lesions compared with those with a single lesion. The main findings of this hospital-based study were that dementia developed through many different locations of multiple lesions involving anterior and posterior circulation. Minor neurocognitive disorder due to multiple lesions is the topic of another study conducted by our group.
The presence of brain microbleeds and the leukoaraiosis burden (especially grades 2 and 3) were found to have a strong association with dementia. This prominent effect of microbleeds and the leukoaraiosis burden in univariate and multivariate analyses may indicate their contribution to dementia in some multiple lesion subtypes. Contrary to our finding, a recent prospective study did not find any evidence of a threshold effect for the leukoaraiosis burden or the number of microbleeds (
26). Our results and previous findings suggest that leukoaraiosis and brain microbleeds make a contribution, to a certain extent, to dementia in the stroke population (
9,
10,
27).
Overall, our data indicate that large-artery disease and cardioembolism are the most prominent factors for poststroke cognitive disorders. This finding emphasizes the fact that these pathologies play a key role in the occurrence of dementia by causing multifocal emboli involving either bilateral or multilevel structures. Small-artery disease was not a significant factor for poststroke dementia in the multivariate analysis. However, limited and strategic lesions in small subcortical structures (e.g., the bilateral paramedian thalamus, caudate nucleus, left arcuate fasciculus, left middle frontal gyrus, and left postero-inferior cerebellum) have been reported as the main determinants of poststroke cognitive impairment, explained by disruption of cortico-subcortical pathways (
28–
31).
There are some limitations to this study. First, in a recruitment time period of approximately 15 years, there was noteworthy progress in the MRI techniques and software to investigate brain structures and lesion volume (
32,
33). We found that stroke lesion volume was likely larger in the multiple infarcts, but we did not determine cerebral atrophy, which could be assessed by using three-dimensional T
1-weighted images with fully automated software, which is one of the most controversial MRI markers with regard to the influence on poststroke cognitive impairment (
26,
34,
35). The mechanism of poststroke brain atrophy has not been well understood, but postischemic secondary neurodegeneration in the subcortical region is probably one of the main contributors (
36). Second, multivariate analysis showed that small lesions (<15 mm) were not associated with dementia either in patients with bilateral infarcts or in those with multilevel infarcts. However, small lesions were visually determined by an experienced neuroradiologist, and improper measurements would have decreased the strength of the relationship with dementia. Moreover, a risk of uncertainty in the measurement of small lesions using specifically automated software methods has been reported (
32). Third, we did not assess cortical microinfarcts, which may be an independent risk factor for cognitive disorder in older populations (
37). Fourth, we used a global mental assessment test to explore cognitive performance. Global mental scores are significantly lower among individuals with multiple lesions compared with those with a single lesion. However, the present study was restricted to the presence of dementia, and we did not analyze subcognitive domains, such as language, executive function, and memory functions, as continuous variables. The association between multiple ischemic lesions and cognitive domains requires further investigation. In addition to the traditional vascular risk factors examined in this cohort, other contributing factors, such as obstructive sleep apnea (
38), hyperhomocysteinemia from common cobalamin deficiency in the elderly, and genetic (
MTHFR and
CTH gene polymorphisms) and dietary factors, are potential causes of multiple infarcts associated with dementia.
This study has many strengths. First, the large sample size enabled us to define many different multiple infarct patterns and feed many factors into the regression model. Our study provides an original and reliable approach using detailed clinical mental status examination and neuroimaging examination to assess multiple and multilevel involvements of brain structures in a large stroke population. Second, we analyzed most of the likely radiological determinants of poststroke cognitive performance, and we delineated the importance of multiple lesions in the anterior and posterior circulation. Third, the study showed a strong association between strategic sites (i.e., the thalamus; angular gyrus; basal ganglia, including the caudate nucleus and globus pallidus; middle and inferior frontal gyri; parietal region; and middle temporal gyrus or postero-inferior cerebellum) and dementia, which may be an independent risk factor for cognitive impairment. Specific clinical pictures have been defined previously in patients with multiple ischemic lesions involving strategic regions either in one hemisphere or in both (
12,
13). A large body of evidence suggests that lesions in strategic areas play a key role in the occurrence of neurocognitive disorder, regardless of their volume (
26), but the associations between strategic site and cognitive domains warrant further investigation that are outside the topic of our study. Fourth, we chose to study only ischemic lesions so that our study cohort was representative of the homogeneous pathology of the clinical population. The study design prevented us from specifically analyzing stroke subtypes, such as subarachnoid hemorrhage and spontaneous cerebral hemorrhage-related lesions. Furthermore, this design has the advantage of reducing multiple linearity and proving the factors associated with ischemic stroke subtypes. Fifth, we found a strong association between the ApoE ε4 allele and dementia, supporting epidemiological association studies showing that the ApoE ε4 allele may increase the risk of dementia, particularly in patients with Alzheimer’s disease or mixed dementia (
39). Last, the present cohort was characterized by an older age and optimal severity compared with individuals from population-based stroke studies (
40). It is well known that demographic characteristics may affect the prevalence and mechanisms of cognitive deficits, with a higher prevalence of Alzheimer’s disease in the oldest patients. Additionally, acute multiple lesions in older patients may cause dementia, depending on the depletion or deterioration of the cognitive reserve.
Finally, multiple acute spontaneous ischemic lesions in the anterior circulation, posterior circulation, or both may be independent factors for vascular dementia. Global cognitive assessment and determination of multiple and multilevel structural involvements related to specific causes are important factors that will determine the cognitive state after stroke.