Sexual dimorphism refers to the differences between genetic males and females within the same species. It manifests in multiple anatomical trait differences, including morphological features and structures of the human brain (
1). Sexual dimorphisms in the human brain were initially reported in the early twentieth century, beginning with the observation that, on average, male brains are heavier and have a larger circumference than female brains (
2). In the intervening years, sexual dimorphism in total brain volume have been broadly described in the literature (
3–10). On average, total brain size in males is about 8%–15% larger than in females (uncorrected for body size) and this sexual dimorphism has been observed from early development studies, childhood, adolescence and adulthood (
3–8,
10–13). Even after correcting for brain volume differences, sexual dimorphism in size-by-age trajectories of brain development (as distinct from group averages across broad age ranges) are observed in frontal, temporal, and parietal gray matter, among other structures (
5). Sexual dimorphisms in the human brain also are associated with differences in behaviors, including courtship, mate seeking, and even aggression (
14). These types of behaviors are instinctual and do not require any specific prior training; instead, they are manifestations of sexual dimorphism in brain development, differentiation, and network function (
14).
Sex steroids (i.e., androgens, estrogens and progestins) are intimately related to the development and further regulation of sexually dimorphic structures, somatic functions, and varied behaviors throughout life, including brain structure and function (
15). Brain exposure to the sex steroids during critical periods of development not only results in structural (anatomical) changes in the brain but also potentiates behavioral changes such as aggression and mating. These hormones program neural circuits to elicit different behavioral responses during adolescence and adulthood upon reinduction from the sex steroids (
16–18). Additionally, sex differences in stress responses follow upon exposure to sex hormones. Those sex hormones, along with their active metabolites, modulate sex differences in stress responses (
19–21).
In humans and other mammals, the brain is wired in a female-like fashion during early stages of neural development. Male-like traits (structurally and functionally) are acquired as a result of in utero exposure to androgens (
17), including fetal testosterone (
22). Synaptic pruning of the medial amygdala is also regulated by sex steroids (
23). Studies indicate that in humans, the third trimester (in utero) is a time of rapid brain growth and development with the total brain size increasing five times its volume (
5,
6). The progression of physiological events during the prenatal period serves to establish the fundamental structure for the development of the nervous system. Intrinsic inputs from within the organism, such as molecular signaling and cross-regional activity, are important as well. Neonatal estradiol (E2) exposure influences microglial cells in the preoptic area of the hypothalamus, thereby regulating the process of masculinization and subsequent male-like behaviors (i.e., copulation, aggression) (
24). The brain continues to grow, while the nervous tissue differentiates after childbirth until age 2. At this age, the child’s brain volume is estimated to be 80% of the typical adult size (
25,
26). Thereafter, the brain further increases its volume during the preschool period, and by age 6 it is approximately 90% of adult size (
8,
26). Further changes in both, gray and white matter tissues will continue during childhood and adolescence. In addition, functional modifications (e.g., cortical, thalamic) occur later (
26).
These processes continue during childhood and adolescence when the entire body and brain undergo important neurophysiological changes in response to the elevated secretion of gonadal hormones during puberty (
27). Additionally, during adolescence there is significant rewiring of the neuronal networks leading to adult cognition acquisition, social behaviors and decision-making mechanisms (
27).These take place subsequent to the remodeling of cortical and limbic system neuroanatomical structures (
27–29).
There are two additional and distinctive ways steroid hormones interact with the brain and effect behaviors: organizational and activational (
28).These principles were first described several decades ago and have been further investigated since that time (
17,
27,
30). Organizational processes potentiate permanent changes within brain structures resulting from the action of steroid hormones during development. These changes persist long after the initial hormonal induction, thereby generating programing codes for important activational mechanisms for steroids during adulthood (
17,
28). Activational processes involve the transient actions of sex steroids on the brain cells in a manner that alter behaviors in relation to certain social stimuli/contexts. The activation is temporary depending primarily on hormonal fluctuations, but influences steroids’ function during later stages of adulthood (
27).
The collective effects of sex hormones on these aspects of neurodevelopment therefore stem from their influences on multiple dimensions of neuronal and glial structure and function. This includes processes such as signaling (intracellular), transcription, modification of transcription (epigenetic), and proteins translation (
31). Accordingly, the effects of sex hormone exposure on human affective behavior are substantial. The individual’s unique experiences (i.e., environment) play an essential role in establishing the mature organization of the neural system (
26). The anatomical and physiological differences of brain development may underlie important risk factors for specific psychiatric conditions for both sexes (
9). Accordingly, the Institute of Medicine (IOM) held a workshop on sex differences and their implications for research in translational neuroscience; they stated: “In the current era of translational research and personalized medicine, it is increasingly important to take sex differences into account, so that the potential effects of products and therapies can be more fully understood” (
32). Later in 2015, the National Institutes of Health (NIH) publicized their expectations regarding “sex” inclusion, as a biological variable, into future research efforts (
33).
Consistent with these calls for research in this area, a growing body of evidence suggests that sex differences in brain development may be one of several factors influencing sex-based differences in the development, age-of-onset, expression, chronicity and course, and treatment response of neuropsychiatric conditions during childhood, adolescence, and adulthood (
4,
5,
9,
12,
31,
34–39). For example, males exhibit higher prevalence of attention-deficit and hyperactivity, autism, and Tourette’s (
5,
40,
41); whereas females exhibit greater prevalence of anxiety, depression and eating disorders (
5,
42–44). In addition, females with mood disorders, such as premenstrual dysphoric disorder, perimenopausal depression and postpartum depression pose outstanding examples of the paramount effects of sex hormones in the brain’s affective regulation. Reports from rodent studies also support this notion (
45,
46). Sex differences in stress‐related anxiety responses also have been reported (
47,
48).
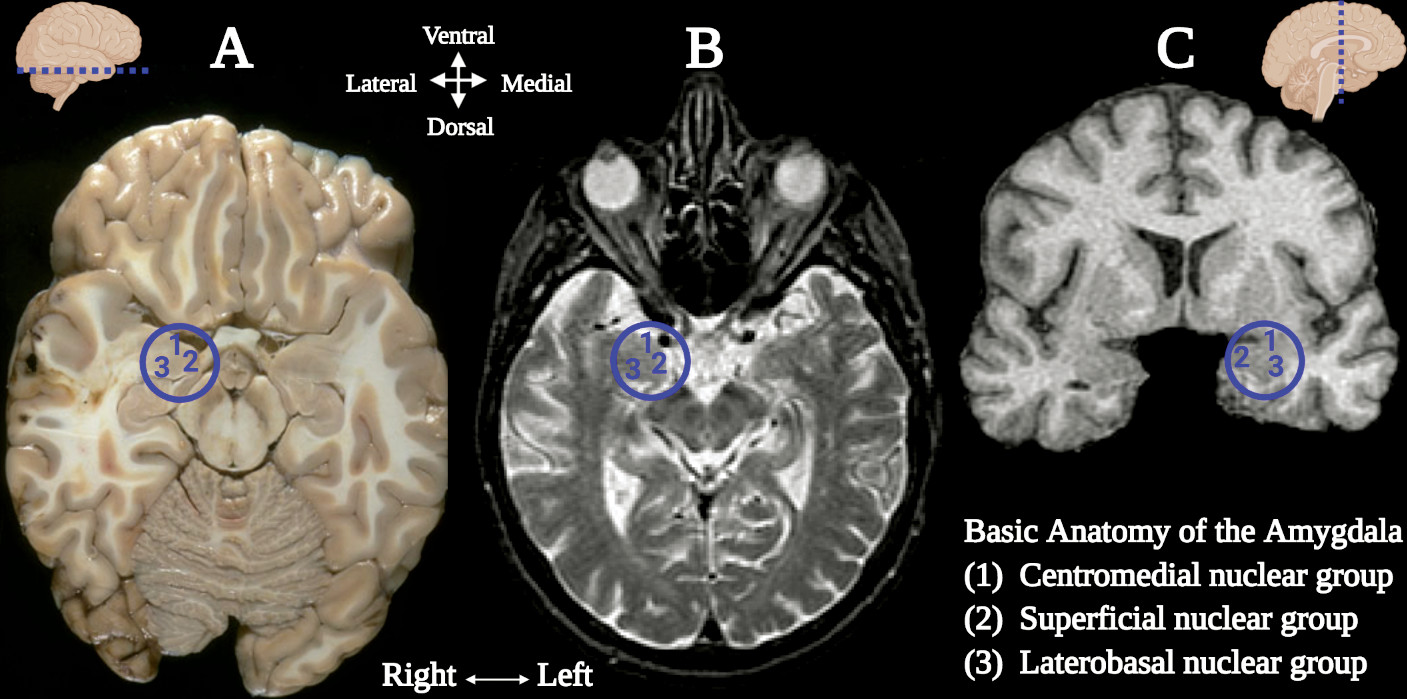
Central to the neural networks involved in these neuropsychiatric conditions is the amygdala, which also demonstrates sexual dimorphism. The amygdala (commonly referred as amygdaloid complex) is a bulky cluster of morphologically diverse nuclei located superior and rostral to the temporal horn of the lateral ventricle. It lies anterior to the tail of the caudate nucleus at the inferior pole of the brain (
49). There are three main regional divisions of the amygdala based on its complex cytoarchitecture and function: centromedial (central and medial nuclei), superficial (ventral nuclei) and laterobasal (basolateral nuclei) (
Figure 1) (
50). The centromedial component is involved with autonomic, endocrine and emotional responses. The superficial group gets input from the olfactory pathways and processes affection. The laterobasal cluster process affective memory and regulates the motor responses associated with fear stimuli (
50,
51). The activation of the basolateral, central, and medial subdivisions of the amygdala elicits anxiogenic effects, while their inhibition produces anxiolytic effects (
49,
52).
Embryologically, the appearance of the amygdaloid primordium is discernible at stage 15 (5 weeks). Whereas the ventral amygdaloid component, the superficial complex and the basolateral complex appear almost simultaneously around stage 16 (five and one-half weeks) (
53). Single nuclear components are noticeable at later steps of development approximately around stages 17–21 (6–7 weeks), and the central nucleus by stage 23 (8 weeks) (
53). During the fetal period, the growth of the amygdaloid complex proceeds with the cellular migration from the ventricular eminences (
54). Subsequent fetal and postnatal topographic changes are attributed to the development and increased growth of the temporal lobe (
53).
Given the potential importance of sexual dimorphism in amygdalar structure and function on our understanding of brain health and disease, the nature and extent of amygdalar sexual dimorphism has been the subject of considerable study using neuroimaging (
5,
55–59). Studies on regional sex differences in the volume of the amygdala have reported mixed findings, some indicating larger volumes in females, while others showed larger volumes in males (
3,
9,
60–63). Other groups have shown decreased or no sex differences when statistically correcting for brain volume (
13,
64,
65). Moreover, a meta-analysis indicated that the amygdala should not be denoted as a sexually dimorphic structure of the human brain (
65). Despite the conflicting results, a number of imaging studies have reported sex differences in the volume of the amygdala even after correcting for brain size (
5,
51,
63,
66–69). An elegant study by Kim and colleagues (
51) evaluated the effects of sex and age within the subregions of the amygdala. They reported that the superficial nuclei showed sexual dimorphism even after correcting for intracranial brain volume, with males exhibiting a larger radius than females (
51). An imaging report (MRI and voxel-based morphometry) from varied brain structures including the amygdala of developing children (37 males and 41 females) between ages 10–14.9 years revealed larger amygdala in males compared with females (
68). A comprehensive study analyzed MRI scans from a sample of 442 developing individuals between ages 8–30 years and reported larger amygdala in males compared with females (
66). Another study of sex differences in brain structure volumes including 643 males and 591 females ages 3–21 years, showed larger amygdalar volumes in males compared with females after correcting for brain intracranial volume (
67). Similar to other brain structures, the overall volume of the amygdala is affected by age. Significant reductions in the centromedial nucleus have been observed in postmenopausal females (
51). Another study by Goddings and colleagues (
69), evaluated MRI scans from 275 individuals studied longitudinally. They reported that males and females have similar overall volume changes in the amygdala as they age, but with different progression paths. The volume of the amygdala rapidly increases in females early in puberty before peaking and decreasing later on. However, in males its volume increases throughout the course of puberty (
5). During adulthood, the amygdala undergoes important structural and morphological sexually dimorphic changes. Its volume decreases more substantively in females as compared with males (
51).
The amygdala is associated with the emotion of fear, among other important functions (
49,
59,
70). Essentially, it is activated during the processing of visual stimuli that transfers emotional meaning during social circumstances, such as the ones conveyed by facial expressions (
Figure 2) (
49,
70). If the amygdala is disrupted, the individual loses the ability to identify the affective meaning of facial expressions. Therefore, the person is unable to recognize threatening facial gestures as presented by others (
49,
70). In addition, patients tend to lose the ability to interpret emotional prosody or the affective contents associated with speech, such as pitch contour, intensity and duration (
49). For example, facial expressions such as staring eyes and furrowed eyebrows, a vocalization using a bold pitch, or the sustained duration of an expressed sentence can lead to fear or anxiety as interpreted by the amygdala (
Figure 2) (
70,
71). Neural activity in response to ambiguous facial gestures of surprise have also been reported to activate the amygdala (
59). However, this activation depends on the individual’s interpretation of such stimuli. If interpreted negatively, the amygdala is activated more. Therefore, higher negative interpretations result in greater neural activity in the amygdala (
59).
When a subject is presented with images depicting aversive facial expressions and strong emotional gestures, regional neural activation in the amygdala and related structures is followed immediately thereafter by an increase in blood flow to those structures (
Figure 2) (
72,
73). Functional MRI (fMRI) and positron emission tomography techniques have been extremely informative in eliciting the functional connection of the amygdala to emotional disorders (
74–77). Such studies have revealed sex differences in the activity of the amygdala, with males demonstrating higher activation in response to sexual stimulus (visual) compared with females (
78,
79). Pleasant emotional stimuli seem to have a sex-specific effect in the amygdala as well. Positive visual stimuli generate greater activity in males, whereas females show higher activation to negative inductions (
80,
81). In addition, there is an age-related decline in the amygdalar activity affecting females more than males. The perception of unpleasant (negative) gestures in elderly females seems to deteriorate more than in male counterparts (
51). This is not unexpected, as the volume of the amygdala is also reduced more in aging females than in aging males (
50).
Sex differences in affective disorders involving the amygdala are consistently reported (
5,
40,
42–44,
82). The literature suggests that males and females experience the same level in the intensity of stress and anxiety, but with some differences that are sexually dimorphic (
48). In general, males are less likely to verbally express signs of distress and anxiety, rather the manifestation of anxiety is internalized physiologically. Males participate more in action‐oriented stress coping strategies compared with females (
48,
83,
84). Seo and colleagues (
48) performed an interesting study using fMRI to examine trait anxiety differences in males and females. The two sexes exhibited opposite paradigms of brain stimulation in response to stress. Females experiencing anxiety during acute stress demonstrated difficulty regulating the increased levels of brain activity. Males manifested anxiety in response to acute stress with a lack of activation or perhaps total inhibition of brain activity during the stressful imagery task (
48).
In summary, sexual dimorphisms in the human brain have neurodevelopmental origins, are modulated by sex hormones, and are further influenced by genetic, environmental, and sociocultural factors in the expression of sex differences in neuropsychiatric health and disease. Among the sexually dimorphic brain structures of greatest relevance to neuropsychiatric disorders is the amygdala, particularly given its role emotional processing and disorders involving disturbances in emotional generation and regulation. However, the consistency and neurobehavioral relevance of sexual dimorphisms in the human brain remain subjects of active study and debate in the clinical and social sciences. Accordingly, further research incorporating methods that explicitly address and meet the calls of the IOM and NIH for study of sex differences and their implications for research in translational neuroscience are needed.