COVID-19 Infection: A Neuropsychiatric Perspective
Abstract
CNS Manifestations of COVID-19
Neurological Manifestations
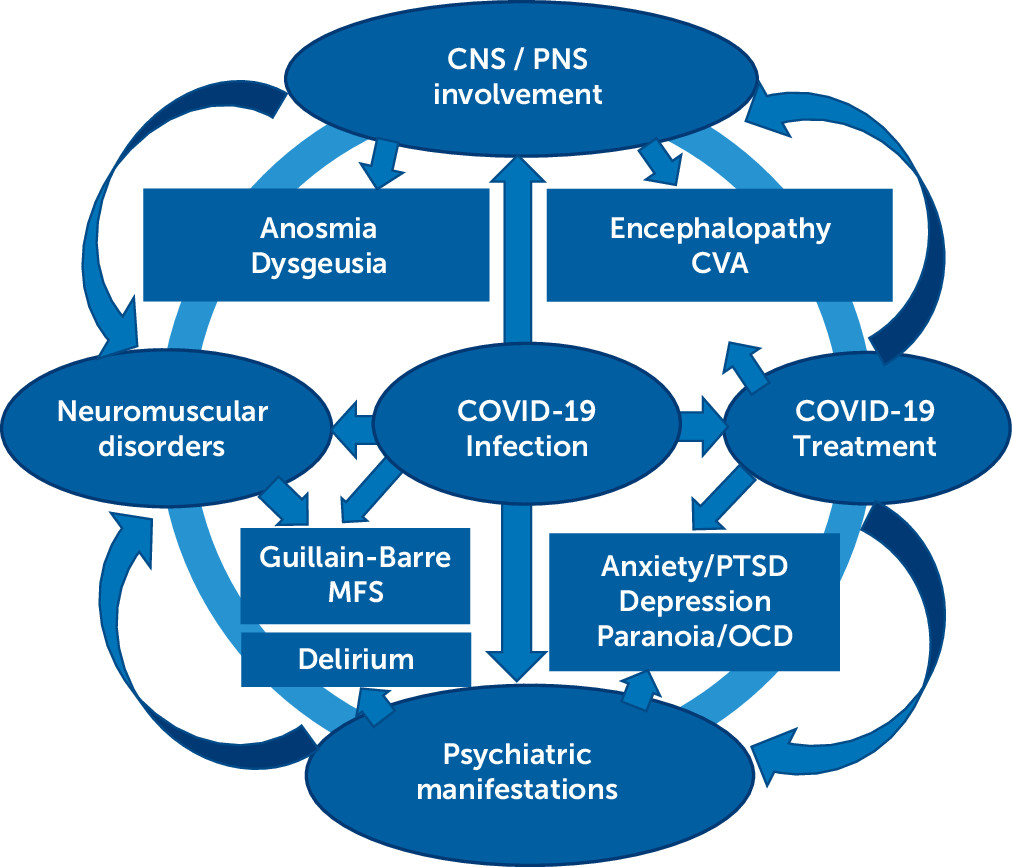
Anosmia and ageusia.
Stroke.
Epilepsy.
GBS and other neuromuscular disorders.
Neuropsychiatric Sequelae of COVID-19
Encephalopathy or delirium.
Neuropsychiatric symptoms and syndromes.
Maladaptive coping styles.
Late Neuropsychiatric Sequelae
Neurodegenerative/Neuroimmunological Disorders
Meta-Analyses
| Study | Number and type of studies | Patients | Outcome | Comments |
|---|---|---|---|---|
| Rogers et al. (8) | 72 (47 studies involved SARS-CoV, 2,068 patients; 13 studies were of MERS-CoV, 515 cases; 12 studies described SARS-CoV-2, 976 patients; 6,390 control subjects) Cohort, cross-sectional, qualitative, case series | 3,559 (patients with SARS, MERS, or SARS-CoV-2) | Patients with COVID-19: delirium: confusion in 26 (65%) of 40 ICU patients and agitation in 40 (69%) of 58 ICU patients in one study; altered consciousness in 17 (21%) of 82 patients who subsequently died in another study. At discharge: 15 (33%) of 45 patients had a dysexecutive syndrome in one study. Two reports of hypoxic encephalopathy. One report of encephalitis. | Patients with SARS or MERS had confusion (27.9%), depressed mood (32.6%), anxiety (35.7%), impaired memory (34.1%), insomnia (41.9%), and steroid-induced mania and psychosis (0.7%). In the postillness stage: depression: 35 (10.5%) of 332 patients; insomnia: 34 (12.1%) of 280; anxiety: 21 (12.3%) of 171; irritability: 28 (12.8%) of 218; memory impairment: 44 (18.9%) of 233; traumatic memories: 55 (30.4%) of 181; sleep disorder: 14 (100%) of 14. Point prevalence of PTSD: 32.2% (121 of 402 patients from four studies); depression: 14.9% (77 of 517 patients from five studies); anxiety disorders: 14.8% (42 of 284 patients from three studies). |
| Wang et al. (9) | 41 (20 reporting unspecific neurological symptoms, 20 reporting specific neurological symptoms, and one reporting both; 26 case series, one cohort study, 14 case reports) | 4,700 (patients with COVID-19) | Anosmia (35.7%–85.6%) and dysgeusia (33.3%–88.8%), especially in mild cases GBS Acute inflammation of the brain, spinal cord, and meninges repeatedly reported after COVID-19. | Possible underlying mechanisms can include both direct invasion and maladaptive inflammatory responses. |
| Deng et al. (10) | 31 (28 cross-sectional studies, three cohort studies) | 5,153 (patients with COVID-19) | Pooled prevalence of depression: 45% (I2=96%); anxiety: 47% (I2=97%); and insomnia: 34% (I2=98%). | No significant differences in the prevalence estimates between genders; however, the depression and anxiety prevalence estimates varied based on different screening tools. |
| Agyeman et al. (26) | 24 (five objective assessments, 19 self-reports) | 8,438 (patients with COVID-19) | Pooled proportions of patients presenting with olfactory dysfunction (41%) and gustatory dysfunction (38.2%). | Increasing mean age correlated with lower prevalence of olfactory (coefficient=−0.076; p=0.02) and gustatory (coefficient=−0.073; p=0.03) dysfunctions. There was a higher prevalence of olfactory dysfunctions with the use of objective measurements compared with self-reports (coefficient=2.33; p=0.01). No significant influence of sex. |
| Hawkins et al. (44) | 229 studies/77 patient reports (37 case reports and case series, 40 observational cohort studies) | 12,971 (patients with COVID-19) | Delirium affected >50% of all patients with COVID-19 admitted to ICU (range: 65%–79.5%). | Higher rates were reported in those with severe respiratory disease (disorder of consciousness: 38.9% versus 7.2%; OR=8.18; acute confusional syndrome: 14.9% versus 3.9%; OR=4.31; p<0.001; confusion: 18.5% versus 0%; p<0.01; impaired consciousness: 14.8% versus 2.4%; p<0.001). Similarly, studies of older adults found that significant proportions experienced delirium while hospitalized with COVID-19, often associated with age and frailty, ranging from 29% to 40%. |
| Tsai et al. (68) | 50 (11 cohort studies, 11 case series, 28 case reports) | 1,326 (patients with COVID-19) | Olfactory/taste disorders: 35.6% Myalgia: 18.5% Headache: 10.7% Acute CVA: 8.1% Dizziness: 7.9% Altered mental status: 7.8% Seizure: 1.5%. | Other manifestations (case reports): encephalitis, neuralgia, ataxia, GBS, Miller-Fisher syndrome, intracerebral hemorrhage, polyneuritis cranialis, and dystonic posture. |
| Brown et al. (54) | 14 (five cross-sectional studies, one survey, one cohort study, one case series, two case reports, three chart reviews, one service evaluation) | 14,465 (one cohort chart review: 13,783 psychiatric patients and 35,909 control patients) | 0.9%–4% incident cases of psychosis in people infected with COVID-19. | Likely associated with steroid or viral exposure, pre-existing vulnerability, and psychosocial stress. |
| Bueno-Notivol et al. (46) | 12 (online questionnaires) | 600–7,236 (non-COVID individuals) | Depression: prevalence rates of 7.45%–48.30%; pooled prevalence of depression was 25%, with significant heterogeneity between studies (I2=99.60%, p<0.001). | Compared with a global estimated prevalence of depression of 3.44% in 2017, a pooled prevalence of 25% is 7 times higher, thus suggesting an important impact of the COVID-19 outbreak on people’s mental health. |
| Ren et al. (47) | 12 (cross-sectional studies) | 27,475 (21,377 general public/6,098 health care professionals) | Incidence of anxiety (25%; 95% CI=0.19–0.32) and depression (28%; 95% CI=0.17–0.38). | Significant heterogeneity was detected across studies regarding these incidence estimates (I2=99.4%). |
| Pinzon et al. (69) | 33 (19 cohort studies, 10 retrospective case series or cross-sectional studies, four case reports) | 7,559 (patients with COVID-19) | Muscle injury or myalgia was the most common (19.2%) neurologic symptom of COVID-19, followed by headache (10.9%), dizziness (8.7%), nausea with or without vomiting (4.6%), concurrent cerebro-vascular disease (4.4%), and impaired consciousness (3.8%). | Most of the included studies were from China: 29 (88%). Underlying cerebrovascular disease was found in 8.5% of the studies. |
| Nepal et al. (70) | 37 (12 retrospective studies, two prospective studies, 23 case reports/series) | 2,647 (patients with COVID-19) | The most commonly reported neurological manifestations of COVID-19 were myalgia (11%–44%), headache (7%–14%), altered sensorium (7%–9%), and hyposmia/hypogeusia (5%–6%). | Uncommonly, COVID-19 can also present with CNS manifestations, such as ischemic stroke, intracerebral hemorrhage, encephalo-myelitis, and acute myelitis; PNS manifestations, such as GBS and Bell’s palsy; and skeletal muscle manifestations, such as rhabdomyolysis. |
| Collantes et al. (71) | 49 (one prospective study, 35 retrospective studies, 13 case reports/series) | 6,335 (patients with COVID-19) | Proportional point estimates (95% CI): Headache: 0.12 (0.10–0.14; I2=77%); Dizziness: 0.08; (0.05–0.12; I2=82%); Headache plus dizziness: 0.09 (0.06–0.13; I2=0%); Nausea: 0.07 (0.04–0.11; I2=79%); Vomiting: 0.05 (0.03–0.08; I2=74%); Nausea plus vomiting: 0.06 (0.03–0.11; I2=83%); Confusion: 0.05 (0.02–0.14; I2=86%); and Myalgia: 0.21 (0.18–0.25; I2=85%). | The most common neurological complication associated with COVID-19 infection was vascular disorders (n=23). Other associated conditions were encephalopathy (n=3), encephalitis (n=1), oculomotor nerve palsy (n=1), isolated sudden-onset anosmia (n=1), GBS (n=1), and Miller-Fisher syndrome (n=2). |
| Abdullahi et al. (72) | 60 (review) 51 (meta-analysis) (46 cohort or cross-sectional studies, four case series, 10 case reports) | 11,069 (patients with COVID-19) | Prevalence of neurological/musculoskeletal manifestations was for smell impairment (35%), taste impairment (33%), myalgia (19%), headache (12%), back pain (10%), dizziness (10%), acute cerebrovascular disease (3%), and impaired consciousness (2%). | The majority (58/60) of the studies had excellent methodological quality. |
| Luo et al. (15) | 62 | 162,639 (COVID and non-COVID individuals) | Pooled prevalence of anxiety (33%) and depression (28%). Prevalence of anxiety (56%) and depression (55%) was the highest among patients with preexisting conditions and COVID-19 infection, and it was similar between health care workers and the general public. | Studies from China, Italy, Turkey, Spain and Iran reported higher pooled prevalence among healthcare workers than the general public. Common risk factors included being a woman or nurse, having lower socioeconomic status, having high risk of contracting COVID-19, and social isolation. Protective factors included having sufficient medical resources and up-to-date and accurate information, and taking precautionary measures. |
| Panda et al. (73) | 26 (21 prospective/ retrospective/case series, five case reports) | 3,707 (children with COVID-19) | Nonspecific neurological manifestations: headache, myalgia, and fatigue (16.7%). Specific neurological complications: 42 children (1%); encephalopathy (n=25), seizure (n=12), and meningeal signs (n=17). | Rare neurological complications: intracranial hemorrhage, cranial nerve palsy, GBS and vision problems. All children with acute symptomatic seizures survived, suggesting a favorable short-term prognosis. |
| Panda et al. (74) | 15 (cross-sectional studies examining the psychological impact of COVID-19 pandemic) | 22,996 (non-COVID children) | Anxiety (34.5%), Depression (41.7%), Irritability (42.3%), and Inattention (30.8%). Behavior/psychological state was affected negatively by the pandemic and quarantine (79.4%). Fear of COVID-19 (22.5%); boredom (35.2%); and sleep disturbance (21.3%). | Caregivers developed anxiety (52.3%) and depression (27.4%), while being in isolation with children. |
Neuroimaging
Neuropsychiatric Adverse Effects of COVID-19 Treatment
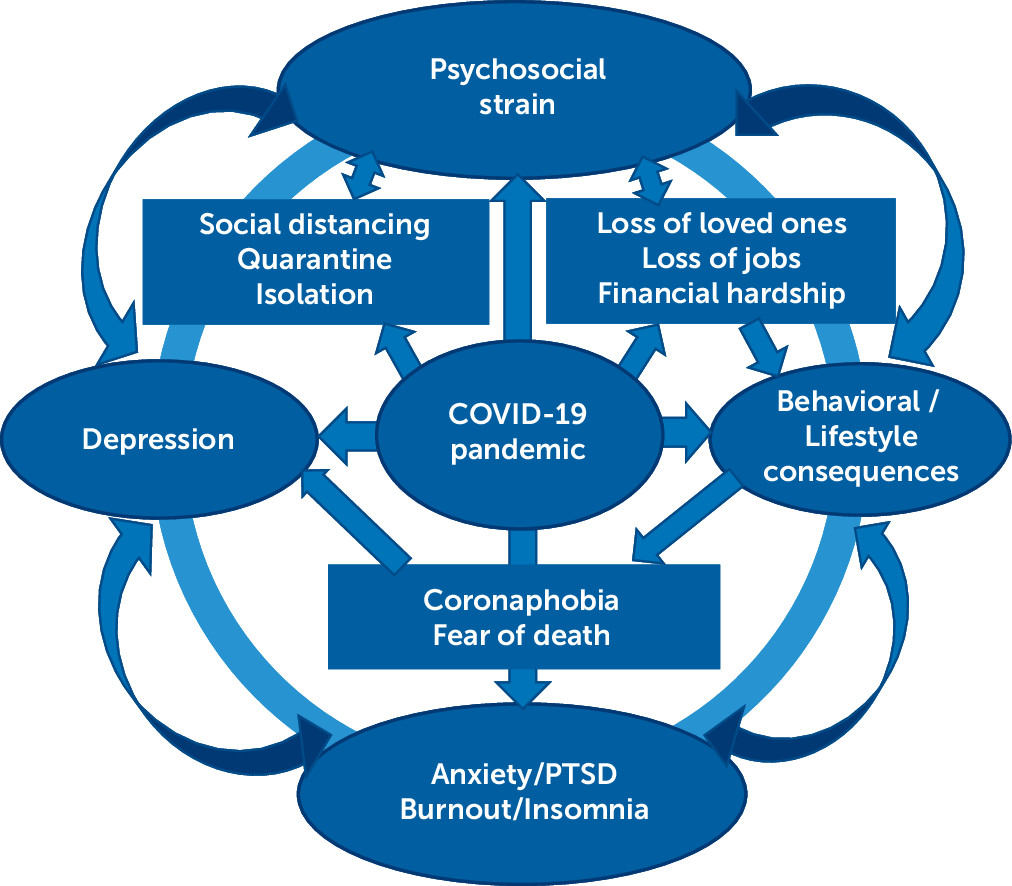
Health Care Workers
Psychosocial Impact
Conclusions
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

