Apathy is a common behavioral symptom of neurodegenerative diseases. The term is derived from the Greek word “apatheia,” meaning without passion. In the 19th century, apathy entered the medical lexicon to describe indifference or inability to feel emotions (
1). Other definitions such as loss of motivation, reduced goal-directed behavior, or a decrease in self-initiation of actions were proposed and subsequently added to the definition (
2). The concept of apathy as a neuropsychiatric syndrome was first proposed by Marin in 1990 in an attempt to differentiate apathy from other clinical disorders. He suggested defining apathy as a separate clinical condition marked by “diminished motivation not attributable to the level of consciousness, cognitive impairment, or emotional stress” (
3). In 1998, Levy et al. (
4) showed that apathy did not necessarily correlate with depression and can be a separate clinical entity across different dementia groups. In the 2000s, research groups attempted to establish a consensus definition of apathy. Starkstein et al. (
5) proposed one of the first diagnostic criteria for apathy, which was later revised by Robert et al. (
6,
7).
Apathy is one of the most common neurobehavioral symptoms of Huntington disease (HD), which is an inherited neurodegenerative disease characterized by progressive movement disorders, cognitive impairment, and behavioral changes (
8). HD is caused by an expanded cytosine-adenine-guanine trinucleotide repeat in the huntingtin gene (HTT) located on chromosome 4 (
9). A recent analysis of the Enroll-HD database found apathy to be the most influential factor in working capacity among those with premanifest HD (
10). Because patients with HD can be unaware of their symptoms (anosognosia), apathy may be underreported yet impose a great burden on caregivers (
11,
12). Moreover, apathy has been strongly associated with the progression and prediction of functional decline in those with HD (
13). Despite being a common and debilitating symptom, the pathophysiology of apathy is still not fully understood, and to date, no medication has been approved for this condition (
14).
In this systematic review and meta-analysis, we aimed to provide a broad perspective on the available evidence regarding apathy in HD, including its frequency in patients with manifest or premanifest HD, its pathophysiology, the clinical instruments used for its assessment, and its association with other clinical domains of HD.
Methods
Design
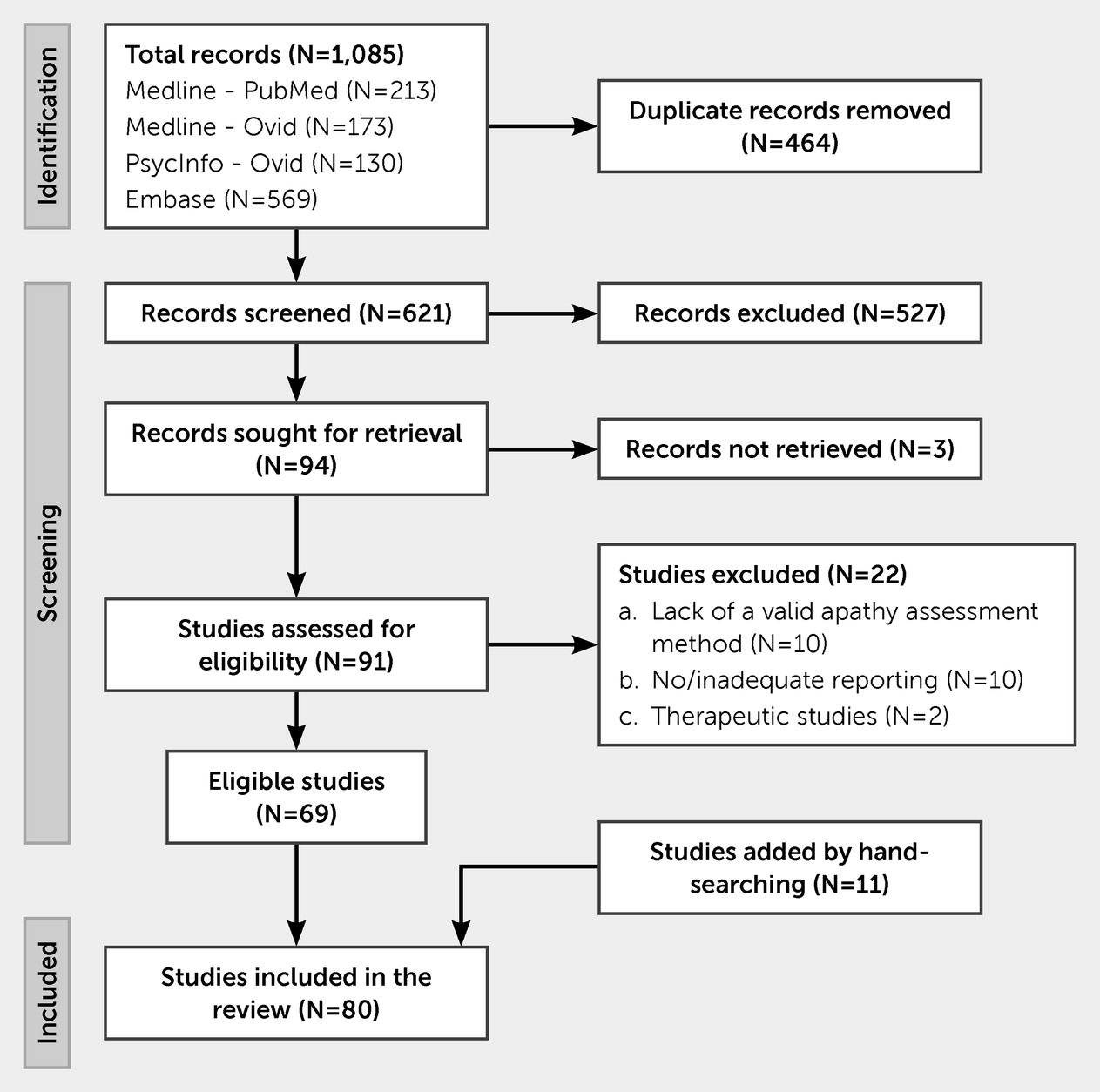
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (
15). A protocol of the study was registered in the international prospective register of systematic reviews (PROSPERO; CRD42022296392).
Search Strategy and Information Sources
A search strategy was developed using the keywords “Huntington disease” and “apathy” and related keywords such as “motivation,” “interest,” “self-activation,” “psychic akinesia,” “athymia,” and “abulia.” A detailed search strategy can be found in appendix 1 of the online supplement to this article. The MEDLINE (via PubMed and OVID), Embase, and PsycINFO databases were searched in November 2021. To include all relevant data, we placed no limitations on the search, including date or language.
Eligibility Criteria and Selection Process
Titles and abstracts were screened by independent reviewers (S.A.Z., H.M.C., and K.S.R.), and the full texts of relevant articles were reviewed for eligibility. Animal studies, case reports, and conference abstracts were excluded during title and abstract screening. Disagreements were reconciled through discussion or by using the expert view of a third reviewer (E.F.S. or A.L.T.).
We included all studies that evaluated apathy in HD patients. The exclusion criteria were studies that did not use a valid scale for scoring apathy or that used a scale not specific for apathy, such as the Beck Depression Inventory (BDI), which evaluates loss of interest related to depression; studies that did not report the number of patients with apathy or the apathy scores for each group; and therapeutic or interventional studies.
Data Collection and Data Items
A predefined data extraction sheet was used to collect information such as sample size, staging of disease, type of assessment, apathy scoring, and number of cases. The primary outcomes were the apathy scores and the frequency of apathy. The secondary outcomes were the pathophysiology of apathy in HD and the association of apathy with other HD symptoms.
Risk of Bias Assessment
Given the heterogeneity of the included studies and the lack of a unified standardized risk of bias (ROB) assessment tool for various study designs, we designed a tool tailored for this study. We partially used the Newcastle-Ottawa Scale and a quality assessment tool developed by Collins et al. (
16,
17). Our modified criteria contain three domains (selection, outcome, and comparability) and nine questions, with a total score ranging from 0 to 14 (see appendix 2 of the
online supplement).
Data Synthesis
The meta-analyses were performed with Review Manager, version 5.4, in accordance with the guidelines of the Cochrane Collaboration (
18). To extract the data from plots, we used WebPlotDigitizer, version 4.5 (
19). Mean differences and standardized mean differences (SMDs) were calculated when the same or different apathy scales, respectively, were used. To compare different subgroups of HD patients, we synthesized a combined group using the means and standard deviations of each subgroup. Heterogeneity was assessed with Cochrane’s Q test and was defined as an I
2 value >50% or a p value <0.05. We performed each meta-analysis with both fixed- and random-effects models and presented the most appropriate model based on heterogeneity and funnel plot asymmetry.
Discussion
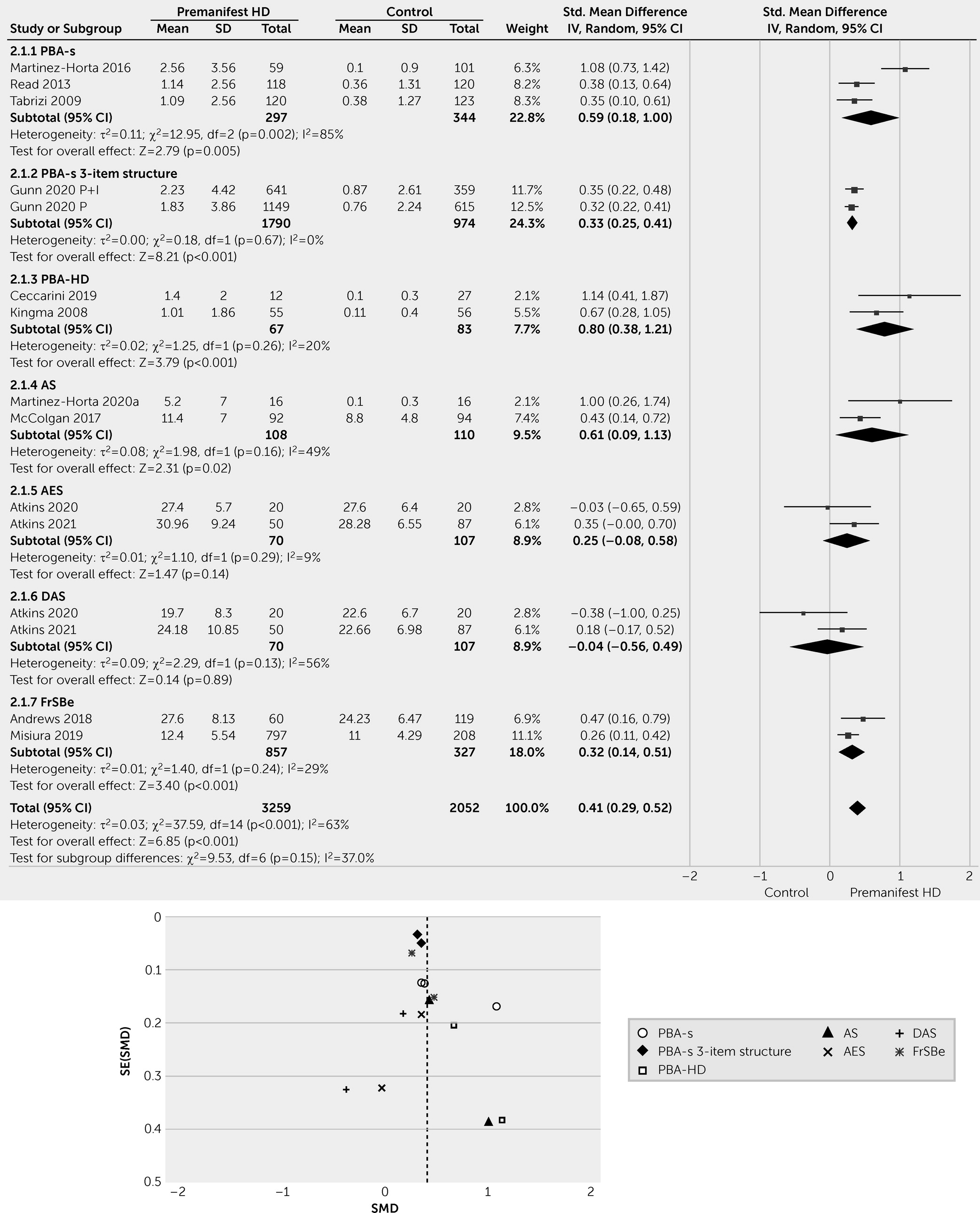
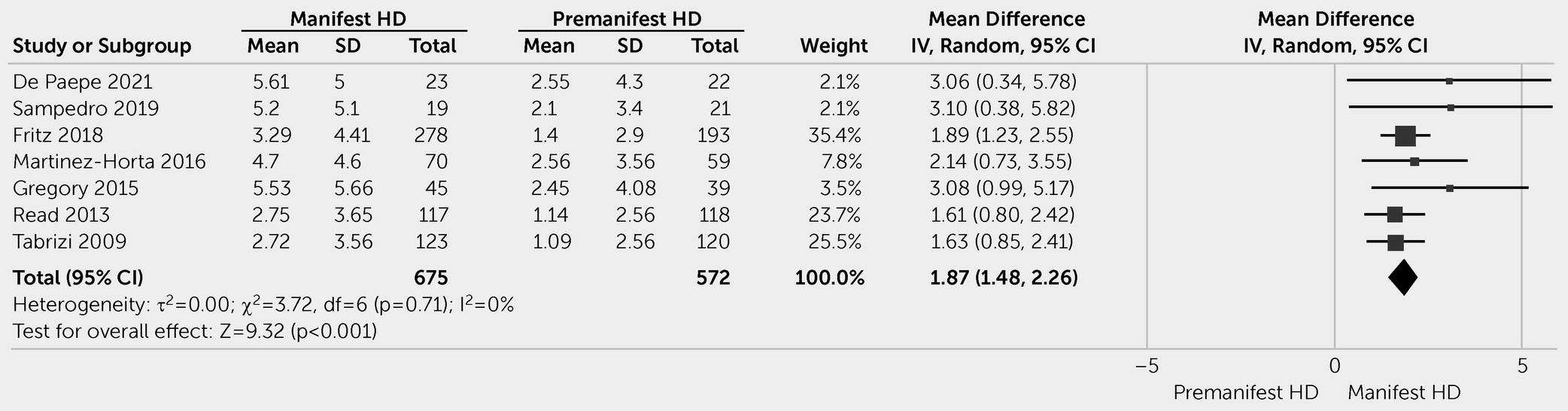
Apathy is a common neuropsychiatric symptom of HD and is associated with disease progression. Our meta-analysis revealed higher apathy scores in patients with manifest HD than in patients with premanifest HD. The heterogeneous data showed that patients with premanifest HD had higher apathy scores than healthy control individuals. The frequency of apathy generally ranged from 10% to 33% among patients with premanifest HD and from 24% to 76% among patients with manifest HD. The numbers varied significantly among studies, reflecting methodological differences (e.g., case definition, assessment tools, source of information, definition of premanifest and manifest HD subcategories) and characteristics of the sample studied, with more advanced HD leading to higher frequency and severity of apathy.
Several instruments have been used to investigate apathy in HD patients. The PBA-s was the most commonly used instrument due to its specific validation in HD and sensitivity to change over the course of the disease (
96). Other apathy scoring tools, such as the AES, AS, and FrSBe (apathy subscale), have not undergone proper validation, specifically within HD patient populations. In the best-case scenario, the validity was assessed in a patient population with a variety of neurodegenerative diseases (
111). Furthermore, differences in the suggested cutoff scores hampered the process of defining a more reliable picture of HD-related apathy. For the PBA-s, for instance, the cutoff scores were defined as severity score of ≥1, ≥2, or >2 in different studies. The reporting of apathy severity was also inconsistent among the studies. PBA and PBA-s scores have been reported in different ways; for example, studies have reported PBA-s apathy severity scores only (range: 0–4), the product of apathy severity and frequency scores (range: 0–16), the PBA-s three-item structure (apathy, perseveration, and disorientation; range: 0–48), the PBA-HD four-item factor (lack of perseverance, poor quality of work, lack of initiative, and poor self-care; range: 0–64), or the original PBA-HD factor with seven items (range: 0–16).
To overcome these shortcomings, consensus-based diagnostic criteria for apathy were proposed (
7). Although these criteria do not measure apathy severity, they provide a diagnostic structure and thus a more reliable case definition. Four criteria should be met for a diagnosis of apathy: a quantitative reduction in goal-directed activity in comparison to the patient’s previous levels of functioning, symptoms and duration, exclusionary criteria, and severity. Three dimensions were defined for symptoms: behavior and cognition, emotion, and social interaction. The patient should have at least one symptom in at least two dimensions, and the symptoms should be persistent or frequently recur over at least 4 weeks (
6,
7). The applicability of these diagnostic criteria in HD needs to be investigated as well as their interaction with clinical tools. Illustrating the relevance of this latter point, the PBA-s and FrSBe had divergent performances compared to formal psychiatric assessments to identify cases of apathy in HD (
43). Therefore, additional studies are needed to establish gold-standard criteria and scales for the assessment of apathy in HD patients.
In this review, we excluded studies that did not use a dedicated apathy scale or subscale. For instance, the BDI assesses loss of interest as a part of depression and does not assess apathy as a discrete entity. While disorders of motivation, including apathy and anhedonia, are relevant elements of depression, they are neither necessary nor sufficient to define the depressive syndrome. Conversely, it is recognized that there is a clinical overlap between apathy and depression (
7,
27). Since depressed mood is one of the early symptoms of HD and given the therapeutic implications, differentiating depressed mood from apathy is clinically relevant (
7,
67). A better understanding of the overlapping versus divergent trajectories of these two neurobehavioral syndromes is definitely warranted in HD.
Apathy was associated with cognitive decline, affecting both global cognition and executive function. Of note, an inability to program and execute plans—core features of executive function—can result in reduced goal-directed behaviors, a subdomain of apathy, indicating a close link between these two constructs (
21). Associations between apathy and cognitive decline have also been shown in other neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) (
112,
113). Even among cognitively normal individuals, apathy was associated with a twofold increase in the risk of conversion to mild cognitive impairment (
114). Taken together, these findings underscore the importance of apathy as a proxy for cognitive decline.
The association of apathy with irritability in HD can be explained by the involvement of relevant subcortical and frontal circuits. Only one study provided evidence in support of the connection between apathy and irritability (
32). It has been suggested that apathy may mask irritability, resulting in the lack of overt external expression of anger (
32). As anosognosia frequently occurs in HD patients, detection of irritability can be challenging in HD patients with apathy and requires a detailed interview with the patient and caregiver.
A role for a number of brain structures has been consistently reported across different disorders associated with apathy, especially PD and AD; these structures include the ventral tegmental area of the midbrain, ventral striatum, and various parts of the PFC, including the ACC (
110,
115). There are fewer neuroimaging studies evaluating apathy in neurodegenerative diseases such as frontotemporal dementia, progressive supranuclear palsy, and HD than in PD and AD (
115). Although the involvement of structures related to effort-based decision making seems to be a common feature of apathy across all these diseases, further studies are required to define the neural basis and pathophysiology of apathy in HD patients.
Our findings should be interpreted in the context of three main limitations. First, the data involved in the comparisons between patients with premanifest HD and control individuals were very heterogeneous. Second, due to the lack of consensus cutoff scores for apathy scales, we used only apathy scores, not the number of patients with apathy, for the meta-analysis. Third, investigating the potential associations between apathy and other clinical domains (e.g., cognition and social functioning) can be affected by confounding factors that were not well controlled in several studies during their design (e.g., randomization) and analysis (e.g., multivariate strategies).