The prominent early presentation of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) includes fever and respiratory manifestations. However, COVID-19 is a multiorgan infection (
1), and more than one-third of infected patients are reported to have psychiatric manifestations in the acute phase (e.g., symptoms of anxiety and depression) (
2,
3). Furthermore, accumulating data suggest that there are long-term sequelae of COVID-19, a syndrome known as postacute sequelae of SARS-COV-2 or “long COVID.”
The psychological impact of perceived danger, social isolation, and physical discomfort constitutes a challenging experience for COVID-19 patients. Previous studies have shown that the onset of sudden and immediately life-threatening illness can lead to posttraumatic stress disorder (PTSD), and epidemiological studies have demonstrated a higher prevalence of mental health problems among survivors of infectious disease epidemics (
4,
5). Additionally, surviving a critical illness has been associated with posttraumatic stress symptoms (PTSS) (
6,
7).
During the COVID-19 pandemic, the diagnosis of PTSD was reported to range from 7% to 43% of patients (
3,
8–
20). These studies were performed shortly after hospital discharge (
3). Data on long-term PTSS among COVID-19 patients are limited. In this study, we aimed to investigate the long-term psychopathological impact of COVID-19 on survivors in a single center in Miami (an epicenter of COVID-19) with the PTSD Checklist for DSM-5 (PCL-5). We hypothesized that hospitalized COVID-19 survivors would have high rates of PTSS at follow-up.
Methods
Study Population
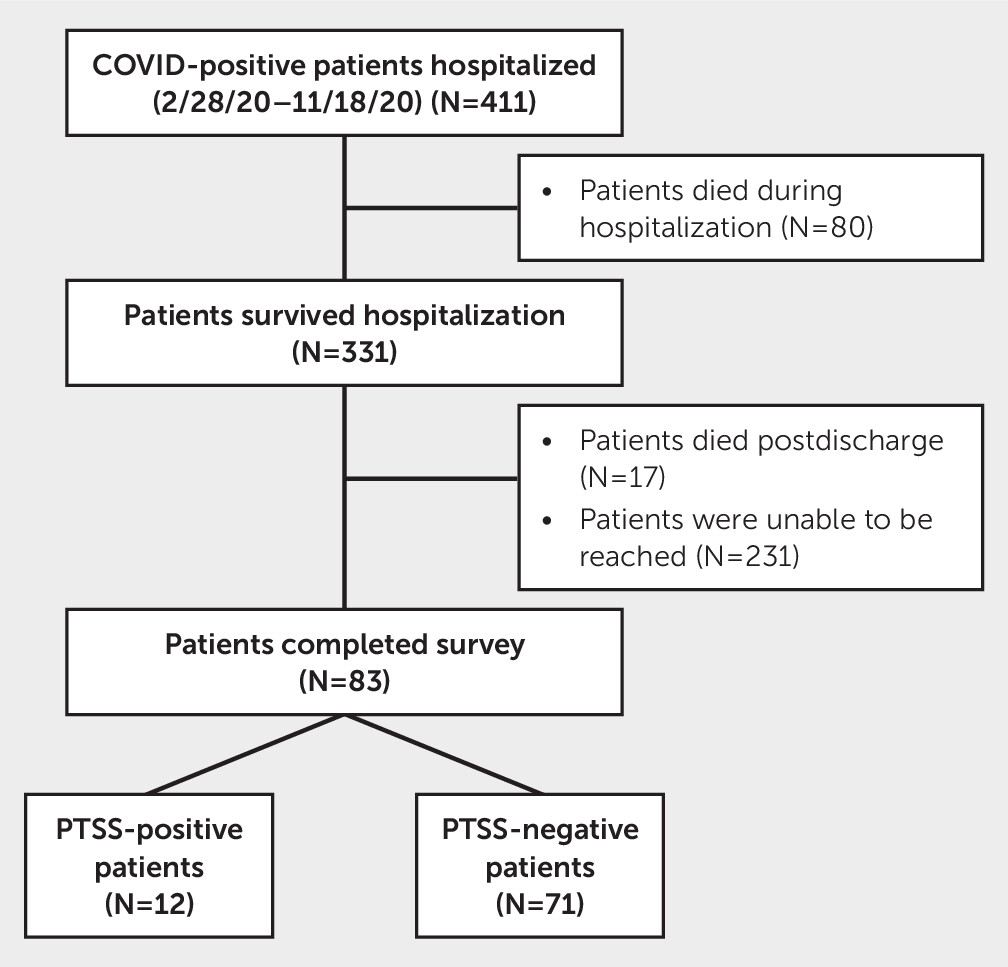
This single-center study was performed at the University of Miami between March 2020 and September 2021 for patients hospitalized between March 2020 and October 2020, a period during which Miami was considered one of the epicenters of the COVID-19 pandemic. A total of 411 adult (>18 years old) patients were identified as COVID-19 positive at the time of hospital admission by chart review. Of these patients, 331 survived to hospital discharge. Following hospital discharge, patients were contacted by telephone at 5- and 12-month follow-up periods. To monitor persistence of symptoms rather than diagnose new cases of PTSS, only those who screened positive for PTSS at the 5-month follow-up were contacted for the 12-month follow-up. At the 5-month follow-up, 17 patients were found to be deceased, and 83 patients completed the follow-up survey (
Figure 1). We excluded patients who survived but were unable to participate due to cognitive or speech impairment, an inability to be contacted, or residence in a long-term facility.
Data Abstraction
Data were collected and stored in REDCap. All baseline and discharge data were collected as part of routine hospital care.
Baseline data.
The collected data included patient demographic characteristics (age, sex, and race-ethnicity), prior neurological and psychiatric comorbid conditions, medical and surgical history, level of care (i.e., intensive care admission status), procedures performed (i.e., intubation, renal replacement therapy, lumbar puncture, tracheostomy, and percutaneous endoscopic gastrostomy), medications used (e.g., vasopressors), and imaging results (e.g., CT, EEG, and MRI).
Discharge data.
Neurological and psychological impairments were determined based on chart review. Discharge disposition was stratified as follows: home, hospice, acute care facility, long-term acute care hospital, or skilled nursing facility. We obtained data on hospital and intensive care unit (ICU) length of stay. Glasgow Outcome Scale (GOS) scores were also obtained at hospital discharge. The GOS was developed to determine the likelihood that patients with acute brain injury would show long-term recovery of daily life and work activities (
21).
Follow-up data (5 and 12 months).
In the follow-up surveys, patients were asked whether they had returned to the hospital and if so, the reason for their return. They were also asked to report on their motor, cognitive, or psychological impairment (based on their belief of their current level of functioning compared with that prior to hospitalization for COVID-19). Cognitive impairment was based on self-reported changes in “concentration, processing speed, language or reasoning capabilities” compared to baseline. Patients were also asked, “Do you feel fatigue?” In addition, the Barthel Index for Activities of Daily Living was used to assess their functional status at follow-up. Finally, a trained research assistant read the PCL-5 over the telephone to patients to assess them for symptoms of PTSD. To minimize loss of follow-up, we attempted to contact each patient three times.
The PCL-5 assesses the presence and severity of PTSD symptomatology in reference to a stressful experience. The PCL-5 is a 20-item screening questionnaire that includes questions that correspond to the four criteria for PTSD diagnosis from the DSM-5: intrusion symptoms, avoidance, negative alterations in cognition and mood, and alterations in arousal and reactivity (
22). In this study, the stressful experience was identified as the patient’s COVID-19 diagnosis and subsequent hospitalization. The scores range from 0 to 80, with a suggested cutoff of 31 for a diagnosis of PTSD. The PCL-5 is, however, a diagnostic screener; in the absence of a clinical interview, PCL-5 scores are not sufficient to make a full diagnosis of PTSD. Thus, we used the term PTSS. The scale answers included “not at all,” “a little bit,” “moderately,” “quite a bit,” and “extremely,” resulting in a score from 0 to 4 for each question asked. A score of 2 (“moderately”) or higher on a question is considered an endorsed symptom (
22,
23). For patients who were screened positive for PTSS (≥31), a contact number for our behavioral health clinic was provided.
Statistical Analysis
The primary outcome was the rate of PTSS among hospitalized COVID-19 survivors at follow-up. The secondary outcomes included a characterization of PTSS-positive patients’ demographic data, events during hospitalization, returning to the emergency department (ED), and follow-up data. Continuous and categorical variables were summarized with means and frequencies (%), respectively. Pearson correlation tests were used to examine relationships between continuous variables, and chi-square tests (or Fisher’s exact tests, when appropriate) were used to examine relationships between categorical variables. Unpaired t tests (or Mann-Whitney U tests, when appropriate) and paired t tests were used to examine independent and dependent group differences for continuous variables, respectively. A multivariate logistic regression model was used to account for age, race, ICU stay, fatigue at follow-up, and returning to the ED and calculated to test the associations between different variables and PTSS status. These variables were chosen for the following reasons: age and race because these are basic demographic variables, ICU stay because it has a known association with PTSS, fatigue at follow-up because it reflects the overall status of the patient, and returning to the ED because it might trigger patients’ stress-related symptoms when reminded of their hospitalization. All analyses were conducted with R software (version 4.0.5), and p values <0.05 were considered statistically significant.
Standard Protocol Approvals, Registrations, and Patient Consent
The University of Miami’s institutional review board (IRB) approved this study. Consent was obtained 5 and 12 months after hospital discharge via telephone follow-up.
Data Availability Statement
Upon reasonable request, the authors are willing to provide deidentified data upon IRB approval.
Results
Of 411 hospitalized COVID-19 patients, 331 (81%) patients survived to hospital discharge. Mean age of the survivors was 66±16 years; 47% were women; 31% were non-White; 69% were Hispanic; 20% were admitted to the ICU; and 11% received invasive mechanical ventilation. There were no differences between the follow-up cohort and either the hospitalized survivor or total population cohorts in age, sex, race, ethnicity, ICU admission, and duration of mechanical ventilation. The only significant differences were GOS scores on discharge, that is, patients who completed follow-up had better recovery than those who survived hospitalization, and the number of patients who received mechanical ventilation (see Table S1 in the online supplement).
Among the survivors, 83 (25%) patients completed the PCL-5 assessment by telephone at the 5-month follow-up. Of those, 12 (14%) patients screened positive for PTSS. We were able to contact eight patients who were PTSS positive at the 5-month follow-up; four of these eight patients remained positive at the 12-month follow-up. Mean age of all patients at follow-up was 62±15 years; 47% were women; 65% were White and 35% were non-White; and 63% were Hispanic. PTSS-positive patients were predominantly non-White (67% vs. 30%, p=0.02), and although the differences were not statistically significant, these patients tended to be younger (56 vs. 63 years, p=0.08) and have shorter ICU stays (2.0 vs. 12.5 days, p=0.06). There were no differences between the PTSS-positive group and the PTSS-negative group in prehospitalization neurological diagnoses (11% vs. 8%), psychiatric diagnoses (17% vs. 21%), or ICU admission (25% for both groups). More patients in the PTSS-positive group returned to the ED (50% vs. 14%, p=0.01), and more patients reported fatigue at follow-up (100% vs. 42%, p<0.001) (
Tables 1 and
2). In the multivariate logistic regression model, non-White race (OR=11, 95% CI=2–91, p=0.01) and returning to the ED (OR=19, 95% CI=3–252, p=0.009) were associated with PTSS-positive status. Differences in PCL-5 scores between the PTSS-positive and PTSS-negative groups, stratified by each question, are presented in the
online supplement (see Table S2).
Discussion
In our racially and ethnically diverse single-center study, we found that PTSS symptoms were common in hospitalized COVID-19 survivors months after infection. In our logistic regression model, we detected a significant association between PTSS and race (i.e., PTSS were more commonly seen in non-White patients) and between PTSS and returning to the ED.
Although many studies have focused on post-COVID depression and anxiety symptoms, few studies have examined posttraumatic symptoms. Most studies were conducted with nonhospitalized patients and relied on the ICD-10 diagnosis of PTSD (
8,
11). These studies showed results consistent with our study with respect to an increased risk of posttraumatic symptoms in COVID-19 patients. In our cohort, 14% of the patients were positive for PTSS, compared with 7%−43% of the COVID-19 population evaluated in other single-center studies, 6.8% of the general population, and 20% of the critical care population (
3,
24,
25).
Most of these studies evaluated PTSD symptoms using the PCL-5 assessment or the Impact of Events Scale (IES-R) (
10,
13,
14,
18,
26,
27). Compared to the PCL-5, the IES-R focuses on three criteria of PTSS (intrusion, avoidance, and hyperarousal) and asks participants to recall how they felt in the past 7 days (compared with the past month with the PCL-5). On average, our study had a longer follow-up than other studies (range: 20 days–4 months).
The population of our study was older than that of other studies. We did not find a significant association between age and PTSS. However, studies with a younger sample reported higher rates of PTSD symptoms (
9,
10,
13,
14,
18,
27,
28). Our sample included 47% women, whereas many other studies included a greater proportion of men (
8,
10,
14,
20,
28). Some studies indicated that male gender was associated with an increased risk of PTSD; others found female gender to be a predictor for developing PTSD (
10,
18). In our study, we found no association between gender and PTSS.
Regarding race and ethnicity, a majority of our patients were White Hispanic. Non-White race was associated with PTSS in our logistic regression model. This is unique, in that most other studies either did not report race and ethnicity or had a predominantly White non-Hispanic population (
3). Our population was ethnically diverse, with 63% of patients in the follow-up being Hispanic; only one other study was found to have a large percentage (70%) of persons from ethnic minorities (
29). Possible hypotheses for this association include cultural differences in the way traumatic experiences are perceived and expressed (
30).
A quarter of our patients were admitted to the ICU, with no significant difference in ICU admissions between PTSS-positive and PTSS-negative patients. Two previous studies found that ICU stay was associated with higher PTSS scores, whereas one study found no association between ICU stay and PTSS (
3,
26).
PTSS-positive patients had a significantly higher rate of returning to the ED after discharge than PTSS-negative patients. PTSS induce a state of hyperarousal in patients, which could create a state in which patients are more likely to return to the hospital for symptoms they would usually dismiss. In addition, PTSD has been identified as a risk factor for acute coronary and cerebrovascular events, as well as other events that might lead the patient to return to the ED (
31).
Our study had several limitations. First, this was a single-center study with a relatively low number of patients, which limits the generalizability of our findings. Second, of the 331 patients who survived to follow-up, only 25% of the patients completed the follow-up survey. Other studies showed similar rates of attrition (
10,
27). Self-selection bias poses a significant obstacle for studies that follow patients after hospitalization. Compared with PTSS-negative patients, PTSS-positive patients reported higher rates of avoidance symptoms (see Table S2 in the
online supplement). This bias is exacerbated by the nature of PTSS because avoidance of reminders of the stressful experience is a symptom. To mitigate this attrition, we attempted to contact each patient three times. Third, positive screening using the PCL-5 questionnaire suggests the diagnosis of PTSD but does not confirm it, and the patients were not evaluated by a psychologist or a psychiatrist. Although this questionnaire is used to screen for PTSD and provides a provisional diagnosis in line with the DSM-5, it is not the gold standard. Given these limitations, future studies can provide a more wholistic understanding of how predisposing factors, hospital course, and discharge disposition affect the development of PTSS. A multicenter study with a larger sample and a longer follow-up would give the medical community more information about the long-term consequences of COVID.