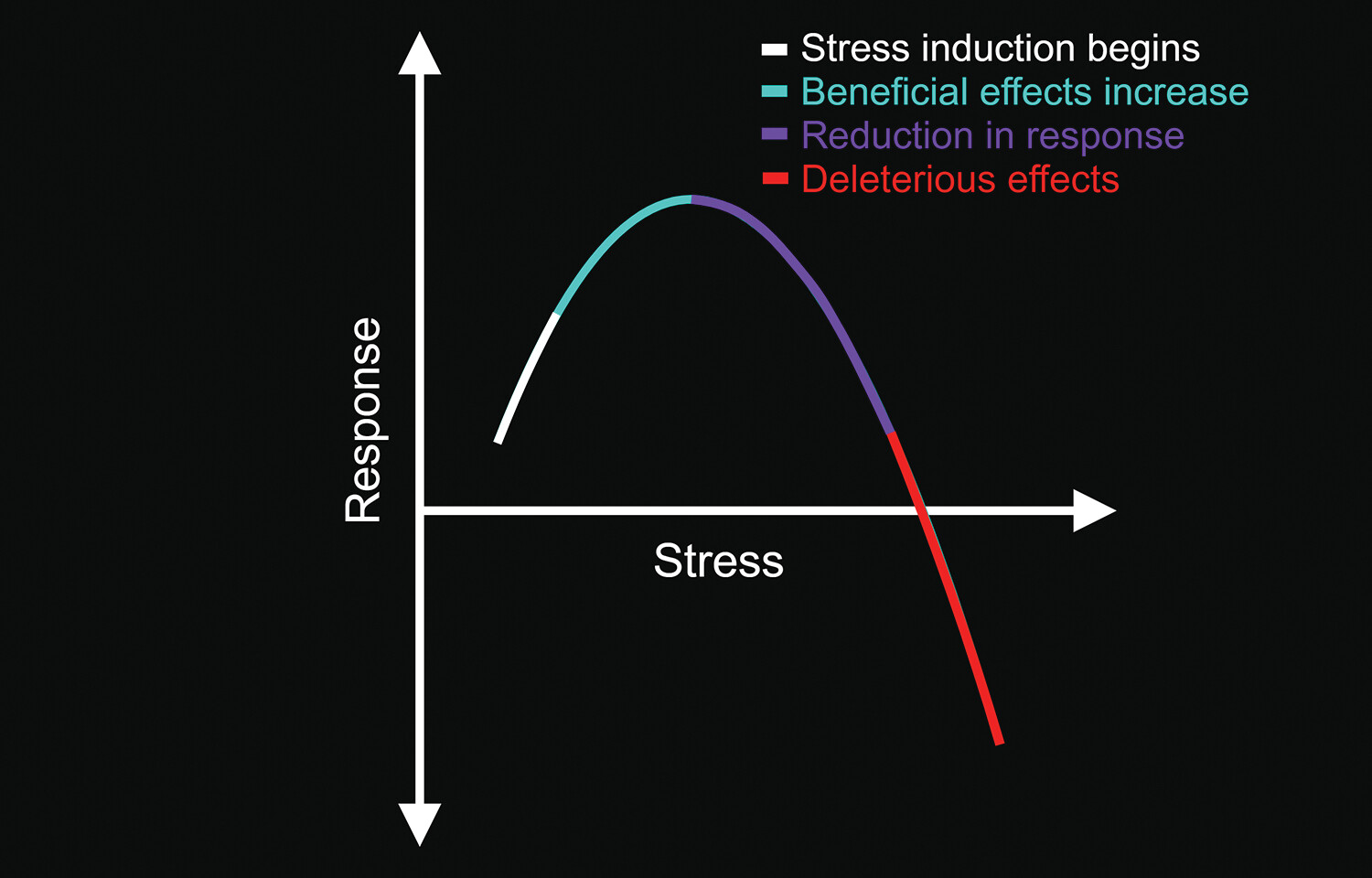
Hormesis is an important evolutionary and physiological concept encompassing stress countermeasures (low-dose stimulation resulting in beneficial effects and high-dose inhibition producing adverse effects), involving well-preserved mechanistic actions and biological plasticity responses (
1,
11,
12). The mechanism underlying the stress response includes both psychological and physiological sequelae to stressor exposure (
2). This biphasic dose response is induced by acute or moderately stressful stimuli (e.g., hormetins, temperature, psychological challenges, and exercise). The induction of this type of stress can yield positive systemic effects (e.g., improved immunological or nervous system responses) and the enhancement of coping mechanisms (
Figure 1) (
1–
5). It is also believed that when one recovery mechanism is induced by stress, other repair and recovery systems have improved function (
13). However, high-level, sustained chronic stress (i.e., toxic stress) produces the opposite effects (
2,
3).
According to health experts, cold-water immersion or a “cold plunge,” a brief dip into a body-size ice bath (10–15°C), produces a wide array of health benefits (
14,
15). This practice originated (in part) from other more natural methods for inducing temporary whole-body hypothermia. For example, winter swimming (e.g., swimming in cold sea water, lakes, rivers, or outdoor swimming pools) is a common practice in countries with cold climates (
16).
Cold-water immersion is an increasingly popular recovery intervention after exercise (
17). Such exposure triggers physiological stress responses with hormetic effects, in which the body is forced to recover its basal core temperature after the cold-water immersion. This response, in turn, produces indirect beneficial effects throughout the entire body (
4,
17,
18). Reports suggest that cold-water immersion can enhance immune system activity (i.e., increase leukocytes and monocytes), increase metabolism and peripheral catecholamine concentrations, increase insulin sensitivity, reduce inflammatory responses and infections, regulate fat metabolic activities, decrease triglyceride concentrations, and reduce stress responses (
16,
17,
19–
22). Furthermore, cold-water immersion or cold-water exposure seems to induce important neurochemical and neurophysiological regulatory processes within the CNS that are linked to mental health benefits (e.g., improved mood and well-being) (
4,
19,
23–
25). Cold-water immersion can also accelerate heart rate, increase blood pressure, and decrease cerebral blood flow.
Basic Neurophysiology of Cold Sensations
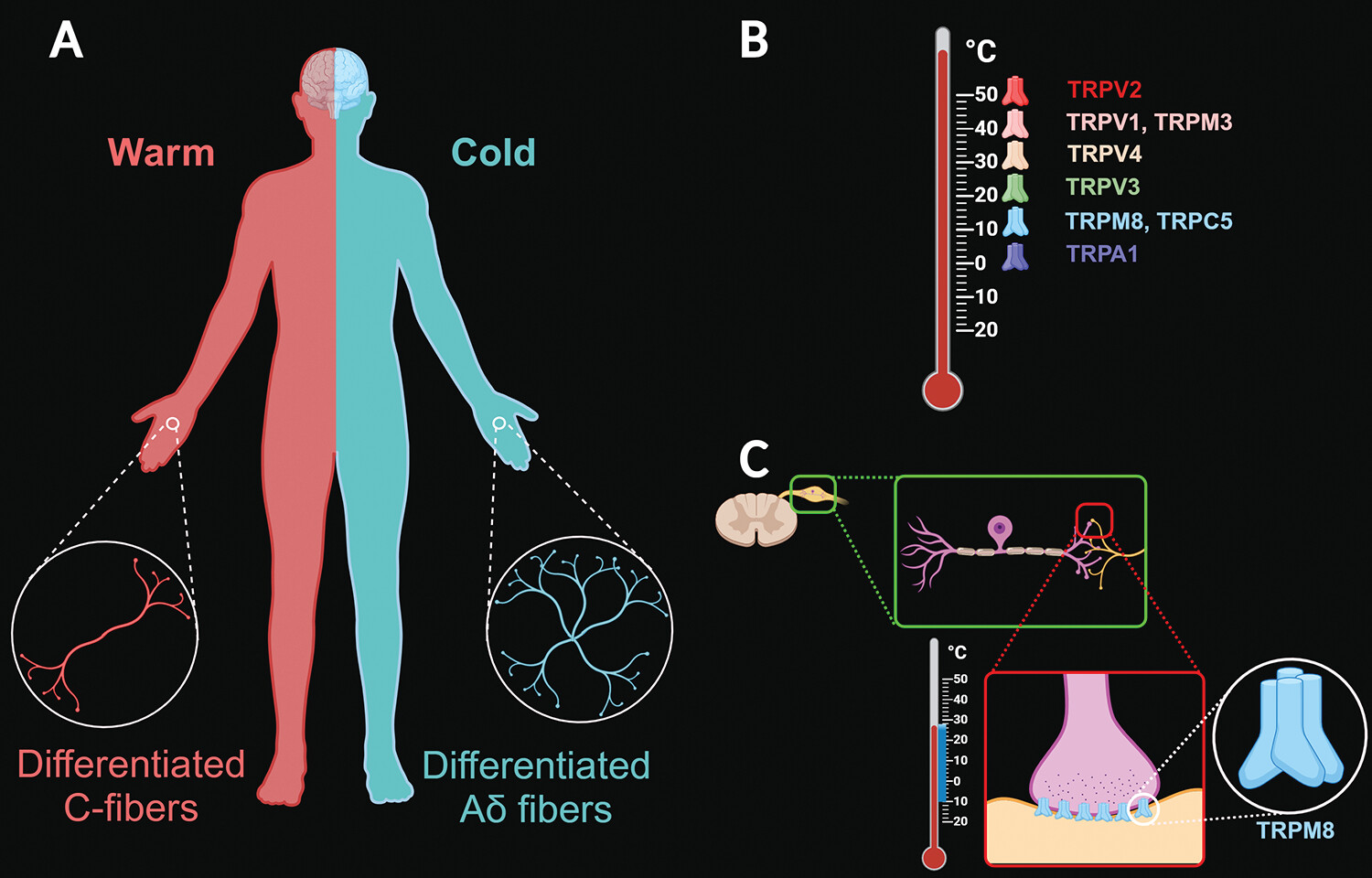
The skin is the largest organ of the human body and functions as the body’s first-order physical barrier from the environment. Various receptors (e.g., thermoreceptors, nociceptors, and mechanoreceptors) are distributed throughout the skin, playing specific roles in the detection of, and adaptation to, changes in the external environment (
6). Separate warm (differentiated C fibers) and cold (differentiated A-delta fibers) thermoreceptors are responsible for temperature sensation. The human skin has a greater density of cold receptors, which varies based on specific anatomical areas. For example, the hands have one to five more cold receptors per square centimeter than warm receptors (
Figure 2A). On the face, the density difference is larger, with possibly 10 or more cold spots per square centimeter for every two warm spots per square centimeter. Similar distribution differences seem to exist elsewhere in the body (
26).
The firing rate of cold-sensing nerves increases at lower temperatures, with the activation of a specific category of voltage-gated ion channels called transient receptor potential (TRP) ion channels. These receptors in the somatosensory system play a pivotal role in the detection of environmental cold temperatures. The various TRP receptors have different physiological properties (
Figure 2B) (
7,
8). For example, the transient receptor potential cation channel melastatin 8 (TRPM8) is involved in calcium ion transmembrane transport and positive regulation of cold-induced thermogenesis. These polymodal receptors reside in peripheral ganglia (i.e., dorsal root ganglion and trigeminal ganglion) and appear to act upstream of or within several physiological processes, including cellular calcium ion homeostasis, responsiveness to temperatures between 10°C and 28°C, cold-induced analgesia, thermoregulation, responses to cooling compounds (e.g., menthol and ilicin), and hyperosmolarity (
Figure 2C) (
7–
10). TRPM8 is the most relevant molecular determinant of cold sensitivity and thermotransduction in primary somatosensory neurons (
10).
Cold hypersensitivity is a clinical concern in certain neurological conditions, such as multiple sclerosis, migraine, spinal cord injuries, and CNS tumors. Thus, TRPM8 is an important molecular target for development of novel therapies (e.g., for pain management) (
8,
10). In addition, certain psychiatric disorders (e.g., depression and anxiety) and other behavioral dysregulations (e.g., fear-associated responses and avoidant behaviors) alter the neuromodulation of calcium ion influx (
27). Furthermore, thermoregulatory responses after cold exposure generate aversive feelings that could mediate behavioral avoidance (
28).
A recent study in mice indicated that TRPM8 is involved in the activation of autonomic hypothalamic neuronal circuits with exposure to innocuous or noxious cold stimuli (
29). The authors also reported that TRPM8 may be involved in acclimation to chronic cold exposure (
30). TRPM8 signaling is essential for behavioral and vascular responses to environmental cold changes, allowing appropriate responses to cold sensations and the conservation of body heat (
31,
32). However, this function appears to be impaired during aging (i.e., middle age), possibly because of reduced TRPM8 gene or protein expression (
32).
Neurohormesis: Neurological and Mental Health Effects of Cold-Water Immersion
During cold-stress exposure, the hypothalamus receives input from central and peripheral thermoreceptors, resulting in activation of the thermoregulatory mechanisms of the body via sympathetic autonomic responses. In turn, increased muscle tone (via the primary motor center) and basal metabolic rate counteract the response to the initial cold-stress stimulus (
33,
34). In addition to these unconscious somatic responses, behavioral responses occur, with which the individual copes by seeking shelter, wearing protective warm clothing, and maintaining physical activity (
35).
Neurohormesis involves important mechanisms relevant to various areas of the clinical neurosciences, including developmental neurobiology, neurology, and psychiatry (
1). Cold exposure triggers the activation of the neuroendocrine axis, increasing the release of neuroactive substances (e.g., hormones, neurotransmitters, cytokines, and growth factors) into the bloodstream and the brain (
4). Mild environmental stressors can activate neuroprotective pathways, decreasing neuroinflammation and neurodegenerative processes, possibly via neurohormesis (
13,
36,
37). In a mouse study, the RNA-binding motif protein 3 (a so-called “cold-shock protein”) was overexpressed in the hippocampus, and both prion disease–infected mice and mice with Alzheimer’s disease mutations showed sustained synaptic protection when exposed to a cooling protocol (
38). If these results are translatable to humans, cold-water immersion could be neuroprotective in neurodegenerative disorders (
19).
Cold-water immersion triggers the release of important hormones and neurotransmitters, such as dopamine, serotonin, cortisol, norepinephrine, and β-endorphins, which are all linked to modulation of the neural responses to stress and other emotion-related circuits affected in depression, anxiety, and posttraumatic stress disorder (
20,
23,
39). In addition, the hormonal changes caused by cold-water immersion seem to help alleviate pain (
20). It has been hypothesized that exposure to cold showers could provide a large-scale stimulus that results in similar effects via activation of peripheral nerves and autonomic mechanisms (
4).
A recent fMRI clinical study showed that cold-water immersion increases the neural interaction between large-scale brain circuits involving multiple limbic structures, including the medial and left rostral prefrontal cortices, left anterior insula, and anterior cingulate cortex. In addition, cold-water immersion facilitated positive affect, including higher alertness and motivation; resulted in a feeling of being more energized (active); and reduced nervousness and distress (
40).
Conversely, the main danger associated with cold-water immersion is the risk of hypothermia, or a decrease in core body temperature below 35°C (
41). Prolonged exposure to cold temperatures impairs memory and decreases attention, affecting response speed (both simple and choice reaction time) and decision making (
42,
43). Cold-water immersion that is severe enough to decrease brain temperature could produce cognitive impairments through alterations in catecholamines and possibly by slowing neuronal conduction and synaptic transmission mechanisms (
42,
44). However, most of the negative effects linked to cold-water immersion are influenced by multiple factors, such as the individual’s general health, including preexisting conditions (e.g., autonomic neuropathies and ganglionopathies); overall level of physical activity; gender, age, body size, and composition; and water temperature, duration of immersion, and frequency of exposure (
19).
In summary, the short stress of cold-water immersion may prime the nervous system with coping mechanisms and thus be beneficial overall. There is also evidence that using cold-water immersion as a form of cryotherapy, especially for body injuries and inflammatory conditions (e.g., rheumatism, traumatic brain injury, and spinal cord trauma), may be helpful. However, these types of interventions remain largely understudied. For instance, the optimal dose of cold exposure has yet to be determined and is most likely to vary among individuals.
Some neurological and psychiatric conditions are linked to dysregulated calcium ion signaling in the brain. TRPM8 channels play an important role in calcium ion transmembrane transport and positive regulation of cold-induced thermogenesis. Thus, effects of cold exposure-based interventions appear favorable.
Preliminary studies seem to indicate that neuroprotective and alternative interventional approaches appear to lessen symptoms in some CNS diseases. One model, the psychoneuroimmunoendocrinology paradigm, encompasses complex interactions between the nervous, endocrine, and immune systems, along with bidirectional networks linking mind and body. According to this paradigm, stress responses can help regulate health and alleviate physiological disease. In this context, neurohormesis may be an interesting homeostatic approach for highly vulnerable biological systems such as the CNS. The hormetic concept is particularly intriguing in clinical neurosciences, considering its potential for both endogenous and exogenous therapeutic responses. Neurohormesis principles (i.e., expression of integrated adaptive responses) may be beneficial in the context of neurocognitive, neurodegenerative, and neuroinflammatory disorders. More clinical trials and definitive studies in this complex area await completion.