The primary role of cranial nerves is to supply sensory and motor innervation to the structures of the head and neck. Cranial nerves comprise nerve fibers associated with different cortical and other brain connections (e.g., brainstem nuclei and thalamic relays) (
4). Compared with spinal nerves, the roots of which are neural fibers from the spinal gray matter, most cranial nerves stem from nuclei functionally organized throughout varying brainstem regions (
4).
The vagus nerve is the 10th cranial nerve (cranial nerve X, or CN X) and the longest of all cranial nerves, originating in the medulla oblongata of the brainstem and extending into the gut region (
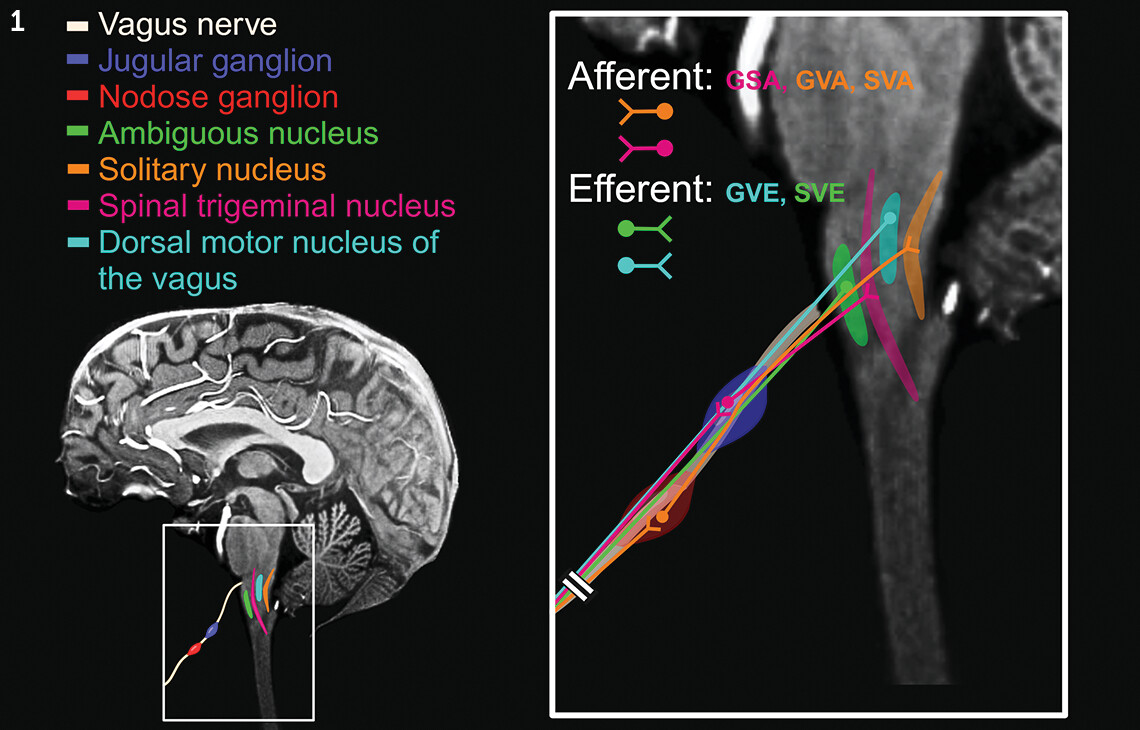
1). It contains two main ganglia (superior and inferior), also known as the jugular and nodose ganglia, respectively (
Figure 1). It exits the intracranial cavity via the jugular foramen, traveling inferiorly and embedded inside the carotid sheath within the anterior triangle of the neck (
1).
CN X consists of both sensory (afferent) and motor (efferent) axons (
1). The sensory axons constitute approximately 80% of the entire nerve bundle. These afferent nerve fibers stem from sensory ganglionic cells located within its two ganglia (i.e., nodose and jugular). The afferent fibers originating from the nodose ganglion terminate in the solitary nucleus, whereas those that originate from the jugular ganglion terminate in the spinal trigeminal nucleus. The neurons giving rise to the efferent axons are located in the brainstem, distributed among the dorsal motor nucleus of the vagus and the ambiguous nucleus (
1,
2). Thus, the CN X is associated with four brain nuclei and two peripheral ganglia, forming important neural circuits (
Figure 1) (
1).
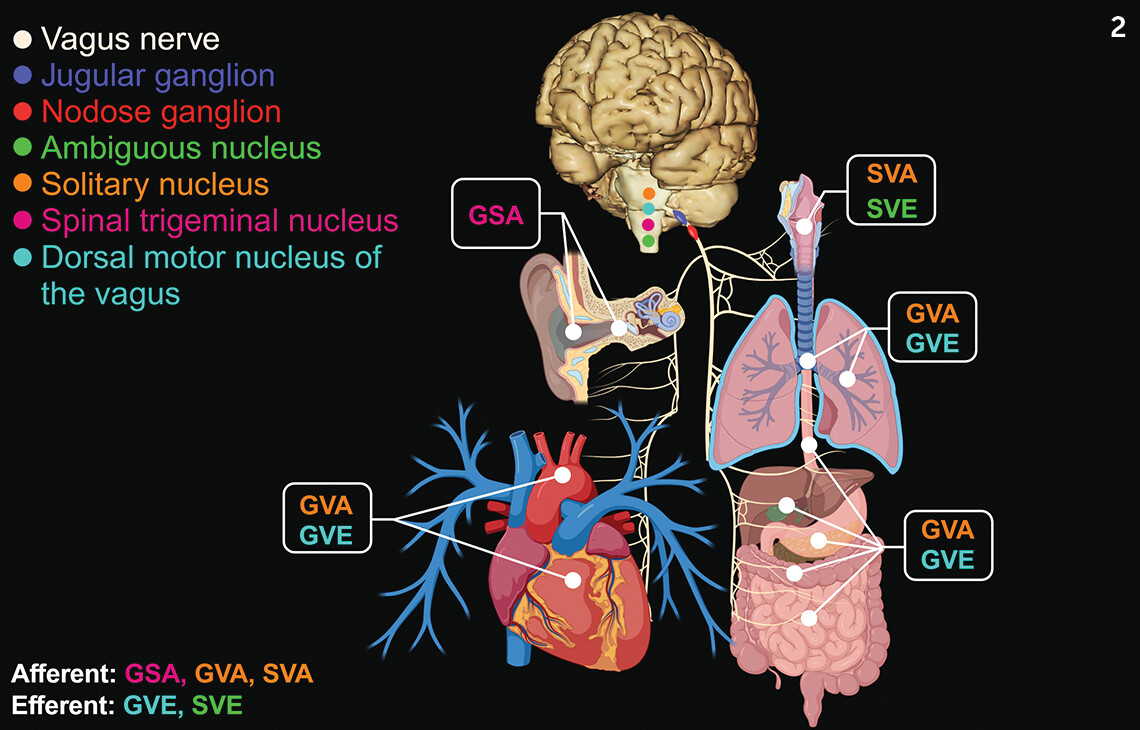
CN X provides the main autonomic (parasympathetic) supply to most abdominal viscera and other organs (
1). It extends the peripheral and autonomic domains of the nervous system throughout the tissues of the gut (via the enteric subdivision of the autonomic nervous system and complex ganglionated, submucosal, and myenteric plexuses), forming a bidirectional neural network that communicates through positive and negative feedback loops (
2,
7).
Visceral Functions of CN X
CN X plays a key role in carrying general visceral afferents (except pain) and special visceral afferents from multiple organs and structures, including sensory signaling and reflexes from chemoreceptors, osmoreceptors, and mechanoreceptors. These signals travel along the vagal sensory branches (central and peripheral), carrying multiple stimuli from digestive system organs, as well as from the heart, lungs, and respiratory tract (
8). The ascending nerve fibers communicate with the vagal sensory neurons in the nodose ganglia, transmitting signals from mechanical stimuli (e.g., distension and rubbing of the gut lumen). They also are influenced by hormones, neurotransmitters, neurotrophic factors, osmolytes, bacterial products, fatty acids, and other nutrients (
2,
9,
10). Neurons in the nodose ganglia project to the caudal aspects of the nucleus of the solitary tract in the dorsal medulla oblongata of the brainstem (
8). The nucleus of the solitary tract is involved in many visceral reflexes, such as the gag, cough, and emesis reflexes (
11). In addition, CN X afferent signaling conveys appetite state, inflammation stages, and other physiologic processes (
Figure 2) (
10,
12).
CN X also carries general visceral efferent fibers innervating the smooth muscle and glandular tissues (exocrine and endocrine) in the upper third of the esophagus, stomach walls, and small intestine as well as a portion of the colon (
9). General visceral efferent fibers also provide parasympathetic innervation to the cardiac and pulmonary tissues. In addition, special visceral efferent fibers supply multiple muscles of the pharynx (
Figure 2) (
9).
Components of the Brain-Gut Axis
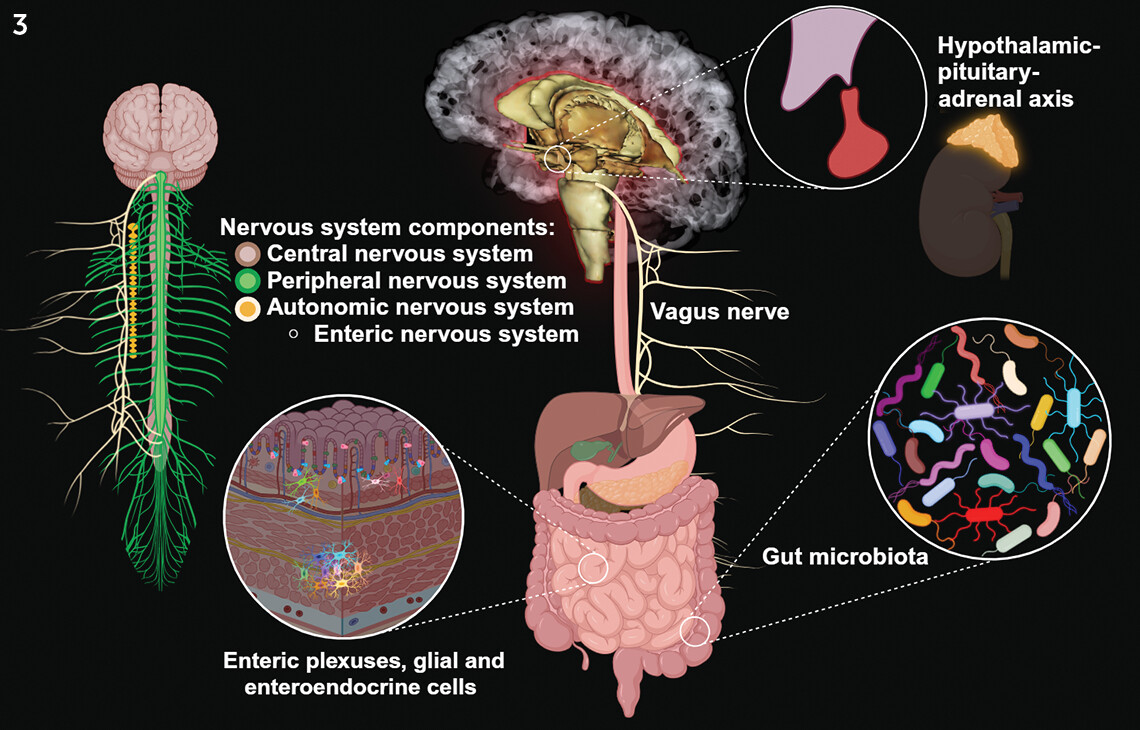
The brain-gut axis (BGA) is a two-way neurovisceral circuit, encompassing the central nervous system (CNS), the peripheral nervous system (PNS), the autonomic nervous system (ANS), the enteric nervous system (ENS) (the so-called second brain), and the hypothalamic-pituitary-adrenal (HPA) axis (
5). Human microbiotas, a population of around 10
13 to 10
14 bacteria distributed among approximately 1,000 species, comprise another component of the BGA (
Figure 3) (
6).
Regarding the BGA, the CN X seems to be the most relevant component of the PNS. It establishes the anatomic connection between the CNS and ENS, potentiating important neural processes, brain functions, and certain behaviors (e.g., feeding and mood) (
1,
4,
13). This neuroanatomic communication system can process, regulate, and condition gut reflexes (e.g., in muscular tissues) as well as adjust to mood variations. Behaviors involved include promoting intuitive decision making, non–associative implicit learning (i.e., habituation and sensitization), redirecting neural signaling processes, and modulating the physiology of the gut, along with other homeostatic and systemic functions (
7,
10,
14,
15).
Despite its length and complexity, CN X cannot reach and supply all the components of the intestinal mucosa directly. However, it can reach them indirectly via its conjoined functions with the ENS plexuses and enteroendocrine cells (EECs) (
2,
7). EECs are specialized epithelial cells that chemically assess the intestinal lumen (neighboring cells and tissues) and its contents (nutritional macromolecules, microbiome, and others), signaling the brain via CN X and secreting important bioactive substances (e.g., cholecystokinin, ghrelin, peptide YY, neuropeptide Y, and glucagon-like peptide-1 and serotonin) (
16). The EEC-vagal circuit is linked to the neuroendocrine pathway of the HPA axis and activated in response to environmental factors, emotions, and stress (
5,
6,
16).
Serotonin is one of the most relevant neurotransmitters in mental health (e.g., depression) and is mostly synthesized outside the CNS by the enterochromaffin cells, a subset of specialized EECs in the gastrointestinal mucosa (
17–
19). These cells regulate the mechanosensory and chemosensory functions that lead to digestion, secretion and absorption, and other metabolic functions (
19).
In addition, EECs functionally interact with enteric glial cells, which further regulate hormone secretions and ENS signaling via the neuroimmune pathway of the BGA (
6). Presently, the study of the BGA and its components has gained significant traction in clinical neuroscience. As a result, a growing body of evidence supports a putative role of the BGA in the pathophysiologic basis of selected mental disorders (
6,
20–
25).
Unique Functions of CN X in Neuropsychiatric Disorders
CN X afferent circuits project to important neuroanatomic structures, including the prefrontal and motor cortices as well as the hippocampus, thalamus, hypothalamus, and insula (
26,
27). CN X modulates the BGA, relevant to a number of neuropsychiatric conditions (e.g., anxiety, depression, posttraumatic stress disorder, Parkinson’s disease, and amyotrophic lateral sclerosis [ALS]), as well as other disorders (
8,
14,
21,
22).
A plethora of scientific evidence indicates that CN X regulates the gut-microbiome-brain axis, playing an important role in the pathophysiology of depression, possibly mediating inflammatory processes between the visceral organs, the gut microbiome, and the brain (
15,
25,
28–
30). A recent study investigated the role of the gut-liver-brain axis in depression-like phenotypes by using mice with liver cirrhosis induced by common bile duct ligations. The intervention caused multiple pathologic manifestations consistent with disrupted gut microbiome balance, depression-like behaviors, and decreased synaptic activation in the prefrontal cortex, among other changes. All these pathologic changes were reversed following a subdiaphragmatic vagotomy (
31). The results indicated that in this model, CN X appears to be involved in regulating the neural mechanism that leads to dysbiosis (changes of the composition or functional alterations of the normal gut microbiome linked to a variety of diseases) among gut microbial species. In addition, depression-like phenotypes improved after a single injection of a new investigational compound, arketamine (
31,
32).
Similar results have been reported with ablated visceral vagal communication in other experiments, resulting in anxiety- and fear-related behaviors, stress reactivity, and impaired cognition, which are linked to brain alterations observed in psychiatric disease (
33,
34). Additional animal studies have shown that CN X–mediated processes are also influenced by the gut microbiome. Some bacterial strains (e.g.,
Lactobacillus rhamnosus JB-1 and
Lactobacillus reuteri) interact with CN X, signaling the brain and altering behavior by reducing stress and anxiety– and depression-related conduct, as well as improving social interaction (
35,
36).
In a preclinical study in mice that underwent fecal microbiota transplantation, inflammatory factors (e.g., interleukin 6, tumor necrosis factor alpha, and C-reactive protein) and intestinal mucosal permeability were increased, as were depressive and anxiety-like behaviors. The administration of probiotics (i.e.,
Clostridium butyricum) ameliorated the depressive and anxiety-like behaviors (
37).
Human studies of treatment-resistant depression show results consistent with the preclinical data (
38,
39). A recent study confirmed that dysbiosis is an important culprit in major depressive disorder, suggesting that the microbiota among patients with treatment-resistant depression significantly differs from that among patients who respond to routine antidepressant treatments (
39).
In addition, there is evidence that proinflammatory species are overexpressed in the gut microbiome of people with Parkinson’s disease (
32,
40,
41). Dysbiosis may result from spreading of systemic inflammatory agents (e.g., cytokines) or altered permeability of the intestinal barrier via a leaky blood-brain barrier and CN X. These problems can generate CNS synucleinopathy that itself potentiates further neuroinflammatory processes in vagal neurons and other brain areas (i.e., in the brainstem and substantia nigra) (
24,
40–
42). The evidence indicates that in Parkinson’s disease, certain dysregulated intestinal pathogen–associated molecules homeostatically expressed in epithelial cells, such as Toll-like receptors 2 and 4, facilitate detection of gut microbial flora and contribute to gut dysbiosis and neuroinflammation (
40,
43).
Gut microbiome imbalances consistent with dysbiosis have been observed in experimental models of ALS and among patients with ALS (
44–
46). This condition is characterized by the progressive loss of motor neurons in the CNS, voluntary muscle wasting, and paralysis (
47). Motor neurons are very susceptible to homeostatic changes and other factors (e.g., stress) (
48). Why gut dysbiosis contributes to ALS remains unknown. However, the metabolic dysfunction, immune dysregulation, and altered gut barrier integrity linked to dysbiosis are potential pathologic mechanisms (
49).
Vagal Nerve Stimulation
Vagal nerve stimulation (VNS) is a neuromodulatory technique approved by the U.S. Food and Drug Administration for the treatment of epilepsy and depression among patients ≥12 years (
50–
53). Clinical evidence indicates that VNS, with implantable and noninvasive devices, can modulate essential functions within the body and treat various systemic and brain conditions, such as treatment-resistant depression and epilepsy (
23,
50–
52). VNS appears beneficial for many patients, including expectant mothers manifesting pharmaco-resistant seizures and children with medically intractable epilepsy (
53). In addition, VNS has shown positive results as an intervention for treatment-resistant depression. A pilot study (N=6) indicated prolonged antidepressive effects after VNS that were linked to the modulation of inflammation responses, relevant neuropsychiatric functions, and blood-brain barrier activity (
51).
VNS appears to induce neural activity (plasticity, modulation, etc.) with positive therapeutic effects. The exact physiologic mechanisms by which VNS exerts its neuromodulatory effects via CN X remain unclear (
23,
27,
54). However, it is believed that VNS potentiates regulation of specific neurotransmitters (e.g., acetylcholine, gamma-aminobutyric acid, dopamine, norepinephrine, and serotonin) and other neuroactive substances (e.g., brain-derived neurotrophic factor and cytokines). These substances are pivotal in multiple brain structures (limbic system, locus coeruleus, cortical and subcortical regions, etc.). They can foster cognition, protect against gut hyperpermeability (preventing gut microbiome translocation), decrease neuroinflammation, promote healthy aging, and facilitate neuronal plasticity (
7,
27,
54,
55).
Conclusions
In the context of the BGA, CN X has a broad role in mental health beyond its wide anatomic distribution and well-known somatic and visceral functions. CN X comprises a direct anatomic connection by which the PNS, ANS, and ENS can communicate with the CNS, integrating all functional circuits of the BGA, including the neural pathway (e.g., CN X, ENS, and neurotransmitters), the immune pathway (e.g., cytokines), and the neuroendocrine pathway (e.g., HPA axis, EECs, and gut microbiome).
CN X–mediated processes are also influenced by bacterial subpopulations in the gut microbiome, positioning CN X as a major conduit for brain health as well as disease. In this role, CN X can alter behaviors (e.g., feeding, satiety, and mood), employing adaptive mechanisms that potentiate homeostasis. However, proinflammatory gut bacteria can transmit inflammatory responses to the brain via the CN X, contributing to serious mental health and neurodegenerative disorders, such as depression, Parkinson’s disease, and ALS.
CN X is also a potential target for innovative interventions for brain disorders. VNS (invasive and noninvasive) is a therapeutic approach that capitalizes on CN X’s neuromodulatory role (via the BGA), inducing neuroplasticity and neuromodulation with beneficial therapeutic effects.
Taken altogether, significant evidence supports CN X’s neuromodulatory role in the BGA relevant to mental disorders. However, many important questions related to the pathogenesis, pathophysiology, and further potential treatments (nerve stimulation modalities, probiotics, symbiotics, and others) for neuropsychiatric and neurodegenerative diseases remain unanswered.