The blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) are exceptional anatomical structures and intricate physiological complexes providing functional and chemical stability to the central nervous system (CNS), including protection from pathogens such as viruses. Despite their extraordinary biochemical and protective efficiency, these structures are considered to be a major target for viral entry into the CNS (
4,
6). Neurotropic viruses are categorized based on their unique tendency for invading and attacking the CNS (
7). A caveat regarding the BBB is that its permeability is not infallible, and neurotropic viruses have evolved to interrupt and evade its shielding properties (
8). A plethora of pathogenic viruses have been identified (e.g., arbovirus, bunyaviruses, cytomegalovirus, enterovirus, flaviviruses, herpes simplex virus, human immunodeficiency virus, human T-cell leukemia virus, influenza virus, lymphocytic choriomeningitis virus, and measles virus). Other neurotropic viruses are the morbillivirus, mouse adenovirus type 1, parainfluenza virus, rhabdovirus mumps virus, parvovirus B19, and most recently discovered, the severe acute respiratory syndrome-related coronavirus-2 (SARS-CoV-2), responsible for the severe pneumonia coronavirus disease 2019 (COVID-19) (
7,
9–
12).
Viral infections affecting the CNS structures are less common than those attacking other body organs (
13). The hematogenous route has been categorized as one of the main pathways for CNS infection (neuroinvasion) for certain viruses (e.g., poliovirus, hepatitis B) (
14). Viremia is a term that describes the presence of viruses in the blood after the virus trespasses the epithelial barrier and spreads to local and distant sites coursing through lymphatics and the bloodstream (
15). The efficiency of the BBB and BCSFB depends on their molecular structure and seamless integrity, restricting the entry of unwanted microbes into the CNS (
4,
15). In the case of the single-stranded RNA genome of SARS-CoV-2, once the virus enters the bloodstream, it binds to the angiotensin converting enzyme 2 (ACE 2) receptors of the endothelial membrane within blood vessels, disrupting the structural and functional composition of the BBB and consequently fiercely accessing the CNS (
16–
18). Other neurotropic invasions occur via the infection of Trojan horse-like immune cells (i.e., phagocytes) that are competent to cross the BBB, thereby transporting viruses into the nervous tissue (
13,
19). Once in the CNS, pathogens are released from the “Trojan horses,” spreading the infection throughout important neural structures (e.g., limbic system, cerebral cortex) (
19).
CNS viral infections may present with acute, latent, or chronic illness, depending on the constant “virtual competition” between the pathogen infection and the immune resistance of the host (
6,
13). However, specific neurological conditions, such as encephalitis and olfactory dysfunctions (i.e., anosmia-hyposmia), posit an interesting argument suggesting a direct invasive route (i.e., retrograde axonal transport) through the olfactory nerve components (e.g., olfactory neuronal axons, olfactory bulbs and tracts). For example, the olfactory nerve (CN I) is considered one of the strongest route contenders for SARS-CoV-2 neuroinvasion due to its proximity to the epithelial lining (olfactory mucosa) within the nasal cavity (
20).
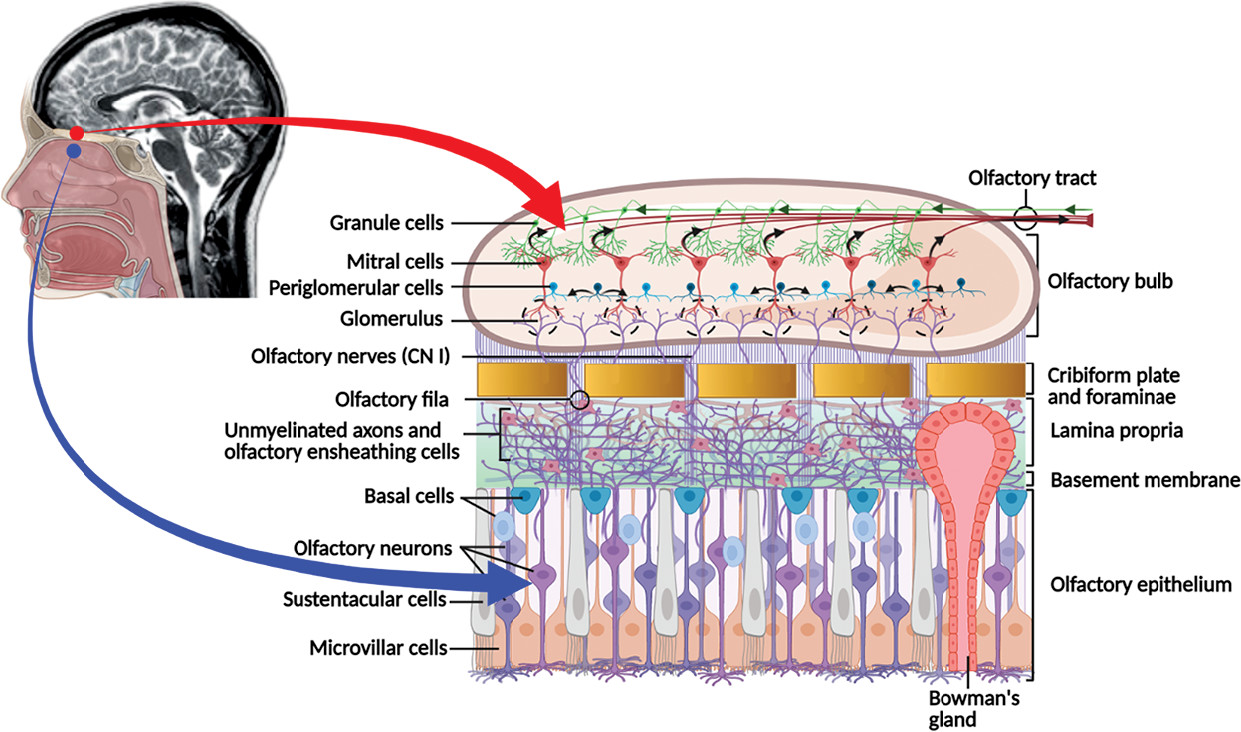
The olfactory mucosa consists of a pseudostratified columnar epithelium composed of varied cell populations, including the microvillar cells (at apical surface), olfactory receptor neurons, sustentacular cells, and two types of basal cells (i.e., globose and horizontal) situated at the base of the olfactory epithelium (
Figure 1). The lamina propria within the subepithelial region consist of connective tissues with blood and lymphatic vessels, nerve fibers (olfactory unmyelinated axons), and the olfactory glands of Bowman’s disease (i.e., branched tubulo-alveolar structures) (
1,
2). The olfactory neuropil (bundles of unmyelinated axons forming the
olfactory fila) originating from the olfactory receptor cells (bipolar neurons) travels through the mucosa toward the cribriform plate of the ethmoid bone in the skull. The small olfactory fila are surrounded by glial-like cells (olfactory ensheathing cells). It pierces the cribriform plate foramina forming CN I and ultimately reaches the glomeruli (approximately 2,000) and the dendrites of the neurons (mitral cells) within the olfactory bulb, where it integrates into varied synaptic connections (
1–
3). The olfactory bulb has a multilayered allocortex structure, such as the cerebral neocortex, but is functionally more complex. The principal cortical neurons are the mitral cells (approximately 50,000) that synapse with the olfactory nerve fibers and give rise to the olfactory tract. The smell signal transduction mechanisms start with the periglomerular cells and continue with the granule cells that refine the odorant information (
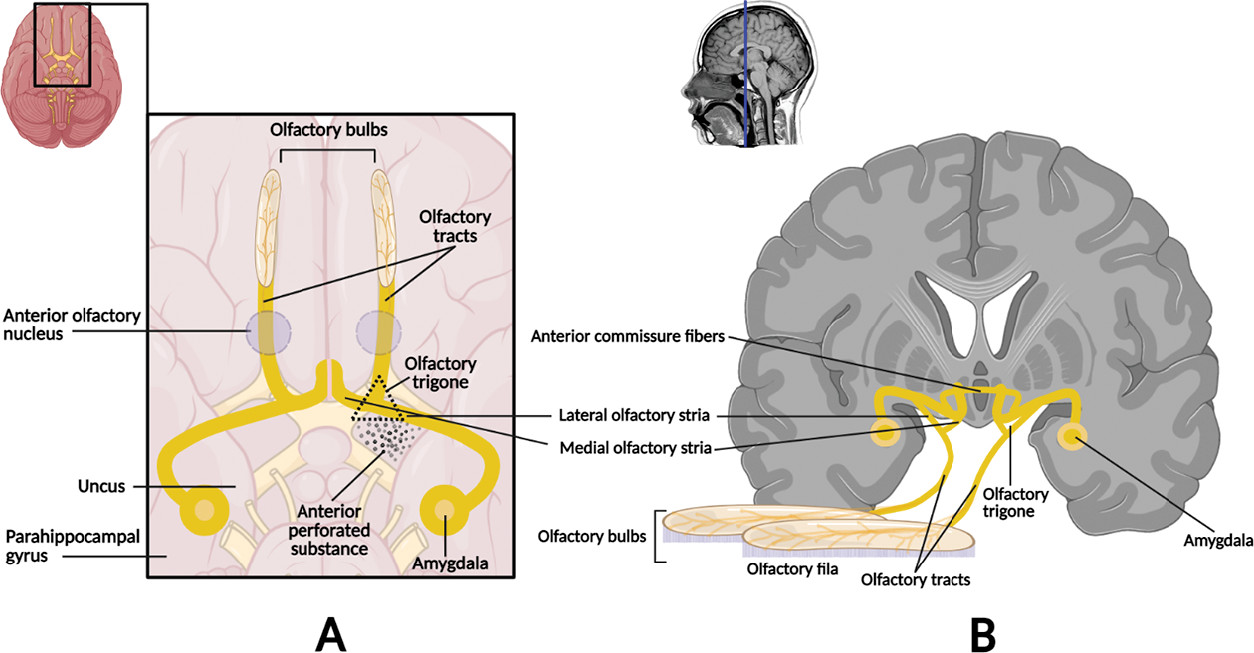
Figure 1). The axons emerging from the olfactory bulb constitute the bilateral olfactory tract, which further divide at the olfactory trigone in front of the anterior perforated substance giving rise to the medial (minor) and lateral (major) olfactory striae. The medial stria contains axons from the anterior olfactory nucleus projecting to the septal area and others coursing the anterior commissure connecting to the opposite tract. The lateral stria terminates in the piriform cortex of the anterior temporal lobe (i.e., ventral portion of the parahippocampal gyrus, amygdala, and uncus) (
4). Altogether, these constitute the main neuroanatomical projections of the olfactory sensory information to varied regions of the CNS (e.g., limbic structures, entorhinal cortex, thalamus) (
1,
4) (
Figure 2).
Interestingly, the cells of the olfactory mucosa, as well as those of the respiratory epithelium, express the angiotensin-converting enzyme 2 (ACE 2) and the transmembrane serine protease 2 (TMPRSS2) receptors, which contain the essential molecular elements for binding, replication, and accumulation of varied viruses, including SARS-CoV-2 (
21–
23). A recent study conducted by Klingenstein and colleagues (
23) using human donor samples demonstrated that ACE 2 and TMPRSS2 are expressed strictly in the sustentacular cells and Bowman glands cells of the olfactory epithelium, however, not in the olfactory receptor neurons (immature or mature) nor in the basal cells of the olfactory mucosa. This important discovery provides further anatomical evidence confirming the histological array of cells expressing ACE 2 and TMPRSS2 in the nose, olfactory epithelium, and olfactory bulb, respectively. It has been theorized that the viral neuroinvasion occurs via the olfactory receptor neurons, thereby affecting their functionality leading to anosmia. However, an important concept to further elucidate is how the olfactory neurons acquire the virus if they are devoid of ACE 2 and TMPRSS2 receptors. An alternative explanation is that either the sustentacular cells or the Bowman gland’s cells (possibly both) may represent the main targets for the SARS-CoV2 virus, considering that they express both of the entry proteins (i.e., ACE 2 and TMPRSS2) (
23,
24).
The sustentacular cells are supporting nonsensory epithelial cells exhibiting an interesting microvilliated apical surface (
2,
3) (
Figure 1). These cells have junctional complexes (i.e., tight and adherens junctions) connecting them directly to the olfactory receptor cells and with other neighboring sustentacular cells (
3). Therefore, they provide physical support, nourishment, and electrical insulation to the olfactory cells and are essential for the structural and physiological mechanisms of olfaction. Sustentacular cells provide mechanical stability comparable to glia cells and perform phagocytosis; thereby they are potentially responsible for protective immunologic mechanisms by expressing antiviral and antibacterial agents (
23–
25). Taken altogether, the viral neuroinvasion of the olfactory receptor neurons may take place indirectly via the sustentacular cells, possibly via their intercellular junctional complexes (
23,
24). This provides insights that could possibly explain the smell dysfunction caused by the olfactory neural pathways disruption, generating long-term anosmia during and after COVID-19 (
22,
23). In addition, the expression of ACE 2 and TMPRSS2 entry proteins seems to increase with age in experimental animals, further aligning (assuming human reciprocity) with the higher infectious risk of SARS-CoV-2 in the elderly population (
24).
In addition to the neurotropic viruses mentioned earlier, varied reports from both human and animal studies indicate that several other viruses can enter the brain specifically via the olfactory mucosa and the nerve fibers of CN I. These include adenoviruses, bunyaviruses, chikungunya virus, herpesviruses, influenza A virus, Japanese encephalitis, La Crosse virus poliovirus, mouse hepatitis virus, parainfluenza virus, paramyxoviruses, rabies virus, vesicular stomatitis, and West Nile virus (
10,
26–
35).
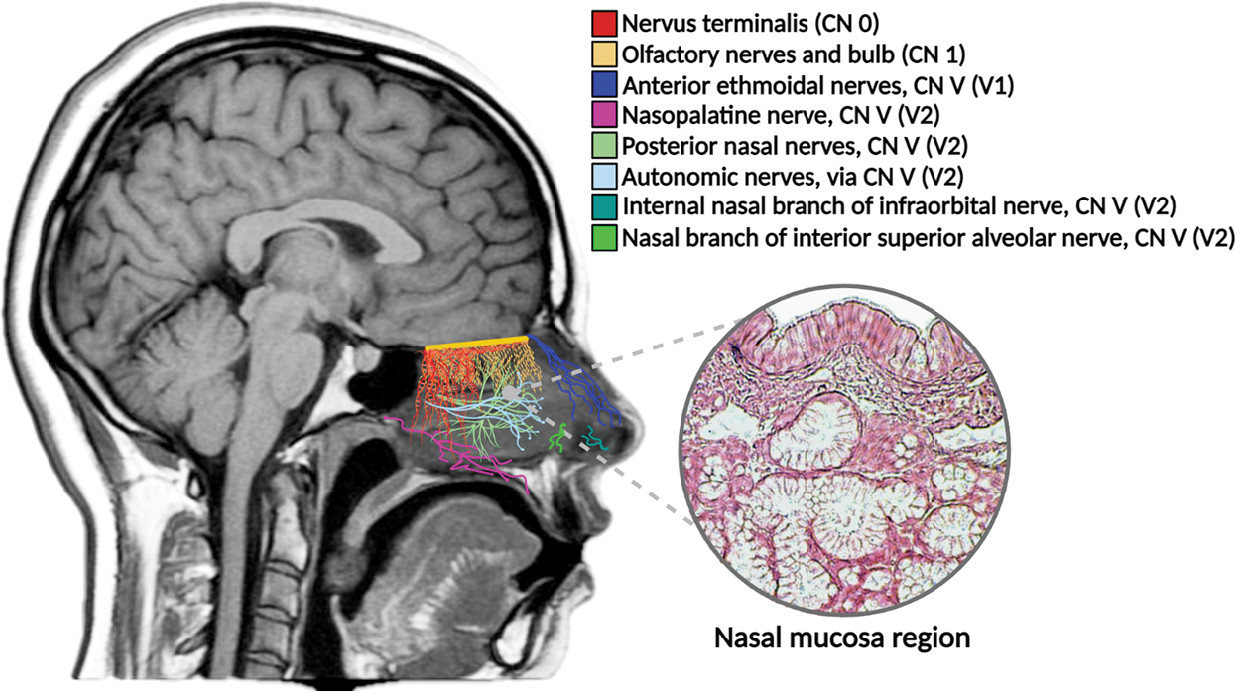
Aside from CN I, there are other important neuroanatomical structures associated with the nasal septum, providing different modalities of innervation to the olfactory mucosa. These include the notable trigeminal nerve (fifth cranial nerve, CN V), the enigmatic nervus terminalis or cranial nerve zero (CN 0), and the autonomic fibers of the cervical ganglia (
1,
5). Given the anatomical location of these structures and their proximity to the olfactory mucosa, they may be potential targets for viral neuroinvasion (
10) (
Figure 3). For example, clinical presentations due to the impairment of CN V and other cranial nerves (i.e., facial nerve, CN VII) have been reported in a significant number of patients with COVID-19 (
36). Herpes zoster ophthalmicus is a viral infection affecting CN V, particularly its ophthalmic division (V1) (
37). During its latent phase, the virus resides within the sensory nerve ganglia of CN V after traveling retrogradely via the CN V axons. During the reactivation phase, it moves anterogradely reaching the superficial tissues of the face where it reemerges with ocular manifestations, including pain, photophobia, reduced vision, and others (
38).
An investigation by Schäfer and colleagues reported (
39,
40) a considerable number of patients with sexual impairment as a concomitant complaint of olfactory dysfunction. More recently, a study showed varied cases of postviral olfactory impairment and reduced sexual function in COVID-19 patients (
41). Normal olfactory functions support the quality and quantity of sexual behaviors (
42). The conscious perception of certain odorants along with the psycho-neuroendocrinological effects of “pheromones” (i.e., odorless social and sexual chemosignal molecules) increase sexual arousal (
43). The CN 0 plays a pivotal role in the maturation of the hypothalamic-pituitary-gonadal gonadotropin-releasing hormone (GnRH) axis, the perception of pheromones, and for triggering sexual responses independently or together with other neural pathways (i.e., kisspeptin neuronal network) (
5,
44–
46). In addition, the CN 0 has been implicated in Kallmann syndrome, a type of congenital hypogonadotropic hypogonadism correlated with various degrees of olfactory dysfunction, consequential to faulty GnRH, and the impaired migration of olfactory neurons (
5,
44,
46–
48).
There is substantial evidence to support the pathophysiological mechanisms for viral neuroinvasion via cranial nerves fibers and other neuroanatomical structures within the sinonasal passages, allowing viruses to enter the brain spreading the infection and affecting the nervous tissue (
10,
36,
49). The CNS sophistication and exquisite neuronal cytoarchitectural arrangement, including its characteristic physiological processes of intracellular conveyance, are regrettably favorable for viral nourishment and replication. The paramount neuronal circuits with their extensive neuropil and widespread synapses somehow facilitate a natural highway for viral infection and neural sepsis (
6). Within the CNS, viruses can cause neurological disturbances, such as induced cell death (i.e., vesicular stomatitis virus), direct lysis (i.e., cytomegalovirus), or indirect injury affecting the release of glutamate, DNA structure, or release of other toxic substances (
13). Viruses such as rabies corrupt cellular transcriptional processes commanding the expression of viral genes within neurons and altering their physiological processes, although leaving their structural composition intact upon routine pathological examination (
13,
50).
The pathophysiological mechanisms of CNS sepsis often include an acute brain dysfunction, typically associated with increased morbidity and mortality rates. The sequelae include inflammatory and noninflammatory alterations of the nervous tissue, resulting in excessive microglial activation, impaired cerebral perfusion, BBB dysfunction, and altered neurotransmission (
51). Unfortunately, one of the most concerning aspects regarding viral infections is that they can produce long-term neurological issues, manifesting years after, or even decades from, the original exposure. For example, the postpolio syndrome (PPS) presents with new clinical manifestations (e.g., limbs paresis, fatigue, arthralgia) in recovered poliomyelitis patients decades after initial polio infection (
52,
53). Some reports have drawn attention comparing the polio epidemics in Europe and the United States during the early 20th century with the current COVID-19 pandemic, warning about a potential disease sequelae (
54). Despite the limited information regarding the long-term outcomes and possible neurologic disturbances of COVID-19, vigilance concerning the chronic effects of viral infections based on the lessons learned from PPS and other viral pandemics is imperative (
53).
From the neuropsychiatric standpoint, another thought-provoking virus is Borna disease virus 1 (BoDV-1), causing behavioral abnormalities and lethal CNS infections in a wide range of species (e.g., cats, chicken, dog, fox, horse, llama, monkey, ostrich, rats, and others), including humans (
13,
55,
56). The virus’ natural reservoir is the bicolored white-toothed shrew (Crocidura leucodon) (
57). The neurotropic BoDV-1 is the prototype of the family Bornaviridae (
Mammalian 1 orthobornavirus). It is an enveloped, non‐cytolytic, non‐segmented, negative-strand RNA virus with slowly replicating capabilities within the nucleus of the infected cells. It can access the brain via the olfactory mucosa and its neural pathways, spread through the limbic system structures to most cortical areas, brainstem, cerebellum, and spinal cord, and remain active in the CNS for years (
13,
55,
58,
59). Although the virus’ relationship with mental disorders was initially established in 1985, the first cases of human encephalitis due to BoDV-1 infection were formally introduced in 2018 (
60–
62). Subsequent reports have indicated a possible link between BoDV-1 infection and mental illnesses, such as bipolar disorder, chronic fatigue syndrome, schizophrenia, and unipolar depression (
61,
63,
64). However, other investigators have argued against any involvement of the BoDV-1 virus in the pathogenesis of these psychiatric disorders (
65,
66). More recently, a systemic review and metanalysis conducted by Azami et al. (
67) demonstrated a possible role of this virus and its potential involvement in the etiology of schizophrenia. In addition, the results from a double-blind placebo-controlled randomized clinical trial study conducted by Dietrich et al. (
68) further supports the established link between BoDV-1 virus infection and depression.
Despite the controversy over the association of certain CNS viral infections with human neuropsychiatric illnesses, neurotrophic viruses posit an intriguing model for the study of psychiatry conditions such as anxiety, depression, and schizophrenia. In addition, peripheral viral infections (nonneurotropic) warrant attention, as their clinical manifestations do not appear to occur from the primary infection. Instead, the secondary neuropathology may manifest much later, presenting syndromic-like clinical scenarios (e.g., Guillian-Barré syndrome, acute necrotizing encephalopathy) (
69).
In summary, infectious diseases are emerging or re-emerging every year, with COVID-19 as the most recent and vivid example. Consequentially, neuroinfectious conditions may occur as outbreaks in small local regions or may spread rapidly over larger geographical territories. Viruses neuroinvasion leads to varied CNS illnesses that may cause a broad spectrum of neurological presentations, such as encephalitis, Guillian-Barré-like-syndromes, meningitis, meningoencephalitis, special senses disabilities (e.g., anosmia, ageusia), and many others. Infections due to neurotropic and nonneurotropic viruses may cause acute and dormant neuropathologic responses; however, some could lead to chronic neuropsychiatry disturbances as a long-term repercussion. It is anticipated that a significant number of COVID-19 survivors may experience innumerable neurological deficiencies and CNS disorders. Thus, an increased awareness regarding the vulnerability of the nervous system to viral infections is critical. It is imperative to increase collective research efforts to improve scientific understanding of the pathophysiology of neurotropism to better prepare for future neurological treatment strategies.