Addenbrooke’s Cognitive Examination–Third Edition Predicts Neuropsychological Test Performance
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Patient Population
| Variable | NC (N=67) | Mild NCD (N=105) | Major NCD (N=45) |
|---|---|---|---|
| Age (M±SD in years) | 71.1±7.4 | 75.0±6.1 | 76.0±7.7 |
| Education (M±SD in years) | 15.5±2.6 | 15.9±2.9 | 15.8±3.4 |
| Gender (N) | |||
| Men | 37 | 57 | 30 |
| Women | 30 | 48 | 15 |
| Handedness (% right) | 95.5 | 90.5 | 75.6 |
Neuropsychological Test Battery
ACE-III Subscales Predicting Neuropsychological Test Scores
| ACE-III subscale and test | F | df | p | ηp2 | Radj2 |
|---|---|---|---|---|---|
| Attention and Orientation | |||||
| WAIS-IV DSB | 2.12 | 8 | 0.040 | 0.13 | 0.07 |
| TMT B | 12.29 | 8 | <0.001 | 0.46 | 0.43 |
| Fluency | |||||
| COWAT | 12.52 | 12 | <0.001 | 0.58 | 0.53 |
| Semantic Fluency | 4.93 | 12 | <0.001 | 0.35 | 0.28 |
| Language BNT | 26.11 | 10 | <0.001 | 0.70 | 0.67 |
| Memory | |||||
| WMS-IV LM I | 3.08 | 16 | <0.001 | 0.32 | 0.21 |
| WMS-IV LM II | 5.61 | 16 | <0.001 | 0.46 | 0.38 |
| Visuospatial | |||||
| WAIS-IV BD | 8.267 | 7 | <0.001 | 0.34 | 0.29 |
Exploratory Analysis: ACE-III Performance by Education
| ACE-III subscale and test | F | df | p | ηp2 | Radj2 |
|---|---|---|---|---|---|
| Attention and Orientation | |||||
| WAIS-IV DSB | 2.78 | 7 | 0.013 | 0.20 | 0.13 |
| TMT B | 15.57 | 7 | <0.001 | 0.59 | 0.55 |
| Fluency | |||||
| COWAT | 8.89 | 11 | <0.001 | 0.57 | 0.51 |
| Semantic Fluency | 4.00 | 11 | <0.001 | 0.38 | 0.28 |
| Language | |||||
| BNT | 23.70 | 8 | <0.001 | 0.71 | 0.68 |
| Memory | |||||
| WMS-IV LM I | 3.55 | 14 | <0.001 | 0.42 | 0.30 |
| WMS-IV LM II | 6.13 | 14 | <0.001 | 0.55 | 0.46 |
| Visuospatial | |||||
| WAIS-IV BD | 7.34 | 7 | <0.001 | 0.40 | 0.35 |
| ACE-III subscale and test | F | df | p | ηp2 | Radj2 |
|---|---|---|---|---|---|
| Attention and Orientation | |||||
| WAIS-IV DSB | 0.81 | 8 | 0.589 | 0.16 | −0.04 |
| TMT B | 0.98 | 8 | 0.461 | 0.19 | −0.00 |
| Fluency | |||||
| COWAT | 8.10 | 12 | <0.001 | 0.75 | 0.66 |
| Semantic Fluency | 1.36 | 12 | 0.249 | 0.34 | 0.09 |
| Language | |||||
| BNT | 9.42 | 10 | <0.001 | 0.69 | 0.61 |
| Memory | |||||
| WMS-IV LM I | 0.90 | 16 | 0.564 | 0.33 | −0.04 |
| WMS-IV LM II | 1.27 | 16 | 0.296 | 0.42 | 0.09 |
| Visuospatial | |||||
| WAIS-IV BD | 1.12 | 7 | 0.372 | 0.18 | 0.02 |
Predicting Neurocognitive Disorders
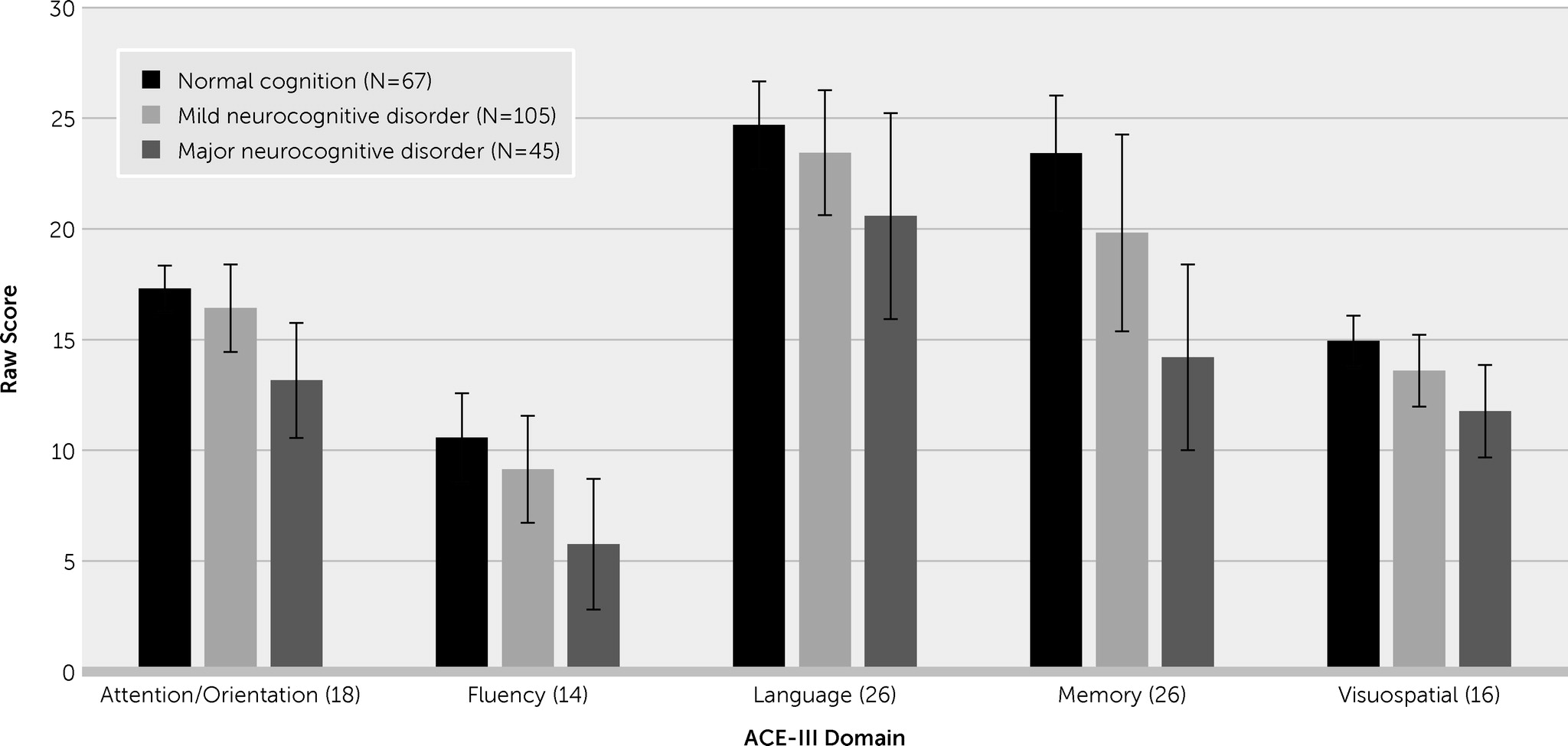
ACE-III Performance by Group
Results
Patient Demographics
ACE-III Subscales Predicting Neuropsychological Test Scores
Exploratory Analysis: ACE-III Performance by Education
Predicting Neurocognitive Disorders
Overall ACE-III Performance by Group
Discussion
Conclusions
Acknowledgments
Supplementary Material
- View/Download
- 82.94 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

