Functional neurological disorder (FND) constitutes motor and/or sensory symptoms that are incongruent with other neurological diseases and instead arise from altered access to voluntary movement control and/or normal sensory perception (
1). FND symptom profiles include seizures, movement disorders, weakness, and sensory abnormalities (
2). Disability among patients with FND can be equal to or worse than that seen among patients with other neurological disorders (
3).
Psychological therapy and physical therapy, either alone or in combination, are the mainstay of treatment for FND and can be effective for some patients (
4–
6). Nevertheless, high-quality evidence supporting treatments for FND is currently lacking (
7), treatment can be difficult to access, and patients with severe illness often require expensive inpatient treatment programs (
8). Even with current best-practice treatments, symptoms often persist or worsen over time (
7). To date, there is limited evidence for the use of pharmacological therapies for FND, although there are specific cases in which they may be effective, such as therapeutic sedation for functional dystonia (
9). There is an urgent need to develop new treatment approaches for this common and highly disabling disorder.
Given the sparsity of effective and accessible treatments, people with FND may seek alternative treatments and self-management strategies, including legal and illicit substances, despite potential risks. Although legal psychoactive drugs, such as alcohol, caffeine, nicotine, and cannabidiol (CBD; a constituent of the cannabis plant), have no proven efficacy in the treatment of FND, their psychopharmacological effects on the central nervous system (CNS) may aid in symptom management, either directly or via an effect on common comorbidities such as anxiety (
10). As an example, the health supplement CBD has been suggested as having promise in the attenuation of symptoms in psychological and neuropsychiatric illnesses (
10).
Illicit substances are used outside of medical settings for control of symptoms in some neuropsychiatric disorders. Cannabis compounds—including smoked cannabis and/or oils containing psychoactive cannabis constituents such as tetrahydrocannabinol—have been used by patients to manage symptoms in conditions such as multiple sclerosis (
11). In a previous online survey of self-management for treatment-resistant cluster headaches, some respondents reported use of illicit substances (cannabinoids, cocaine, heroin, lysergic acid diethylamide, and psilocybin) for acute symptom relief and prophylaxis (
12,
13). Illicit psychedelics have also been used by some in the prophylaxis and treatment of other disorders, such as migraine (
14) and chronic pain (
15).
There has been renewed medical research interest in the medical use of psychoactive substances, such as psychedelics (
16–
18), 3,4-methylenedioxymethamphetamine (MDMA) (
16), cannabis (
19), and ketamine (
20,
21), as treatments for neuropsychiatric disorders. These treatments are often administered as single doses in medically supervised environments, thus avoiding the need to take medication regularly (
16). There have been specific suggestions that ketamine, MDMA, and psychedelics could be interesting research targets in the treatment of FND (
21–
24). Ketamine is a dissociative anesthetic that has similarities to other agents used in therapeutic abreaction, and it has an emerging evidence base in trauma-related pathologies such as posttraumatic stress disorder (PTSD) (
25). MDMA has also shown promise in the treatment of disorders such as PTSD when it is used alongside trauma-focused therapy (
26). Amphetamine has shown promise in the treatment of medical disorders featuring a fatigue component (
27). Additionally, psychedelics may have therapeutic potential in FND, particularly because they may help to retrain somatic self-representation via activation of serotonin 2A receptors in neural pathways such as the default mode network (
22).
In the present study, we collated self-reported views on self-management strategies used by patients with FND through a large online survey. Specifically, we sought to understand the prevalence of legal and illicit substance use, subjective effectiveness, and respondents’ views on novel alternative therapies. We aimed to explore the use of illicit psychoactive substances to manage symptoms, as well as views on the acceptability of these substances as treatments for further research.
Methods
Respondents
Respondents were recruited through relevant groups on social media (i.e., Twitter and Facebook) and patient support groups (e.g., FND Hope, FND Action, and FND FrieNDs). We recruited respondents who self-reported receiving a diagnosis of FND from a doctor; however, the nature of the survey meant that diagnoses could not be clinically verified.
Design and Materials
This was a cross-sectional, observational survey study. An online questionnaire was created and shared, and open access spanned over 1 month (September—October 2019). Questions were written in plain English, avoiding medical jargon where possible. We received support from the charity FND Hope in the design of the survey. Detailed information on the study design, as well as additional results from the survey, can be found in a previously published study (
28). Relevant sections of the original questionnaire are presented in the
online supplement.
Questions were either multiple-choice checkboxes, visual analogue scales, or free-text fields. Basic demographic information was obtained, and respondents were asked about their use of strategies to manage their symptoms, which included legal substances (such as caffeine) and illicit substances (such as cannabis). Respondents were asked to rate the effectiveness of the strategies they had used to manage their symptoms. Finally, they were asked how willing they would be to consider psychedelic therapy if it were found to be safe and effective.
Where respondents were asked to rate the effectiveness of interventions or strategies, the visual analogue scale included 100 increments labeled as follows: “not effective at all” (0/100), “moderately effective” (50/100), and “extremely effective” (100/100). Where respondents were asked to rate the degree of their agreement, the visual analogue scale included 100 increments labeled as follows: “strongly disagree” (0/100), “neither agree nor disagree” (50/100), and “strongly agree” (100/100). Where respondents were given the option to select more than one answer, mutually incompatible answers were removed from the data set.
Data Analysis
Data were analyzed with IBM SPSS Statistics, version 26.0 (Armonk, N.Y.). Descriptive statistics (i.e., frequencies, proportions/percentages, and measures of central tendency and dispersion) were used to summarize the data. Between-group frequencies were compared with use of chi-square tests. Kernel density estimates were constructed with custom scripts on RStudio Desktop.
Ethics
The study conformed with the ethical principles of the World Medical Association Declaration of Helsinki. The study was approved by King’s College London Research Ethics Committee (HR-18/19-11278).
Results
Respondent Characteristics
A total of 1,162 respondents from 16 countries completed the survey. Most respondents (N=851/1,084 79%) resided in the United Kingdom. Most respondents (N=1,048) reported a formal diagnosis of FND from a consultant neurologist or other suitably trained clinician. Those who did not indicate that they had a diagnosis were excluded from the survey and analysis. Females comprised 86% (N=903) of respondents. The mean age of the respondents was 42.5 years (SD=12.7). The mean overall duration from first FND symptom to survey was 8.7 years (SD=9.4), and the mean time from first FND symptom to diagnosis was 5.4 years (SD=8.6). Further details on demographic and illness characteristics of the respondents are described elsewhere (
28). There were minor variations in response rates throughout the survey.
Self-Management: Legal Substances
Prevalence.
Overall, 451/980 (46%) respondents indicated that they had tried legal substances to help manage their FND symptoms. The breakdown of substance use is summarized in
Table 1.
Effectiveness.
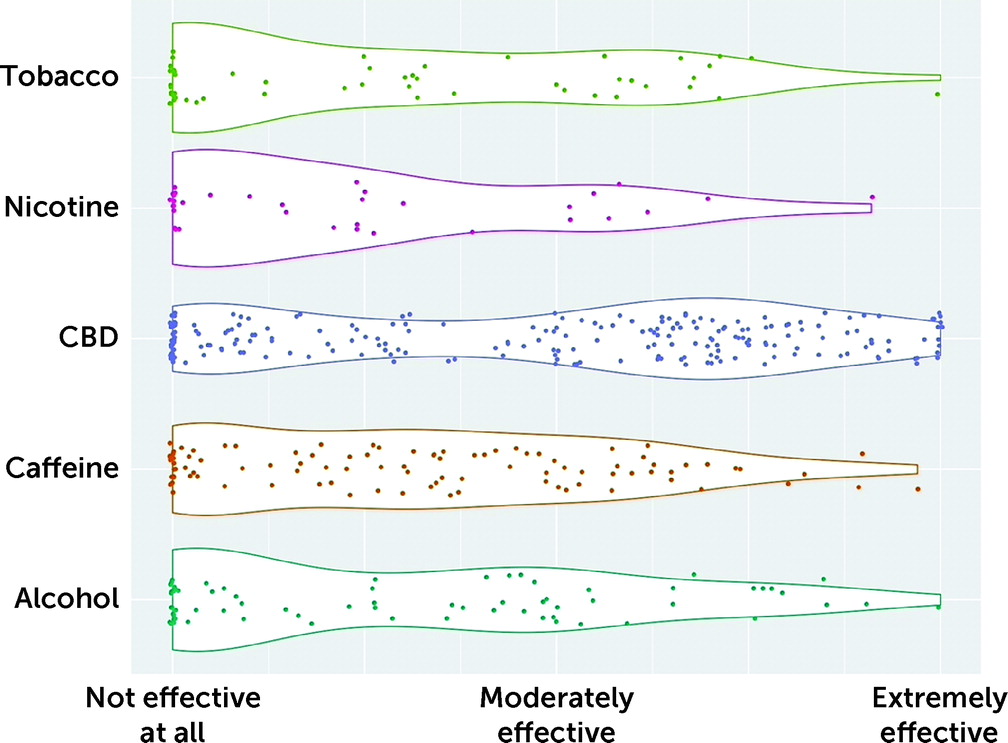
Respondents were asked to rate the effectiveness of legal substances for their FND symptoms on a visual analogue scale. Median values (out of 100) were as follows: cannabidiol (CBD), 52.0 (N=196); caffeine, 34.0 (N=87); alcohol, 32.5 (N=68); tobacco, 22.0 (N=51); and nicotine, 18.0 (N=30). In the case of CBD, 22% indicated that it was extremely effective (>75 on the visual analogue scale). Self-reported effectiveness of each substance in the management of FND symptoms is reported in
Figure 1. Use of caffeine was more likely to be reported among those who currently took prescription medication; however, use of CBD was unrelated to medication status (see Table S1 in the
online supplement).
Self-Management: Illicit Substances
Prevalence of use.
Overall, 79/979 (8%) patients indicated that they had used prescription medication obtained without a prescription (e.g., over the Internet) to manage their FND symptoms. Furthermore, 329/978 (34%) respondents had considered using illicit substances to treat FND symptoms but had not done so because of illegality or concerns about safety. In total, eight respondents indicated that they had been legally prescribed medical cannabis for their FND symptoms.
Illicit substances, such as cannabis, ketamine, and psychedelics, had been used by 151/978 (15%) respondents to self-manage FND symptoms. Some respondents reported that they had used more than one substance. The breakdown of reported use of different substances is summarized in
Table 1. Use of illicit substances was not significantly related to whether respondents had taken prescribed medication (see Table S1 in the
online supplement).
Complications.
Most respondents who had used illicit substances reported experiencing either no or minimal physical (90%) and psychological (95%) sequelae from use. Despite this, all illicit substances were rated as having at least “some” psychological or physical complications (except psychedelics, which had the highest rating of “some” psychological complications and “minimal” physical complications). Cannabis, cocaine, and MDMA were all rated as having severe psychological or physical complications by at least one respondent (
Table 2).
Effectiveness.
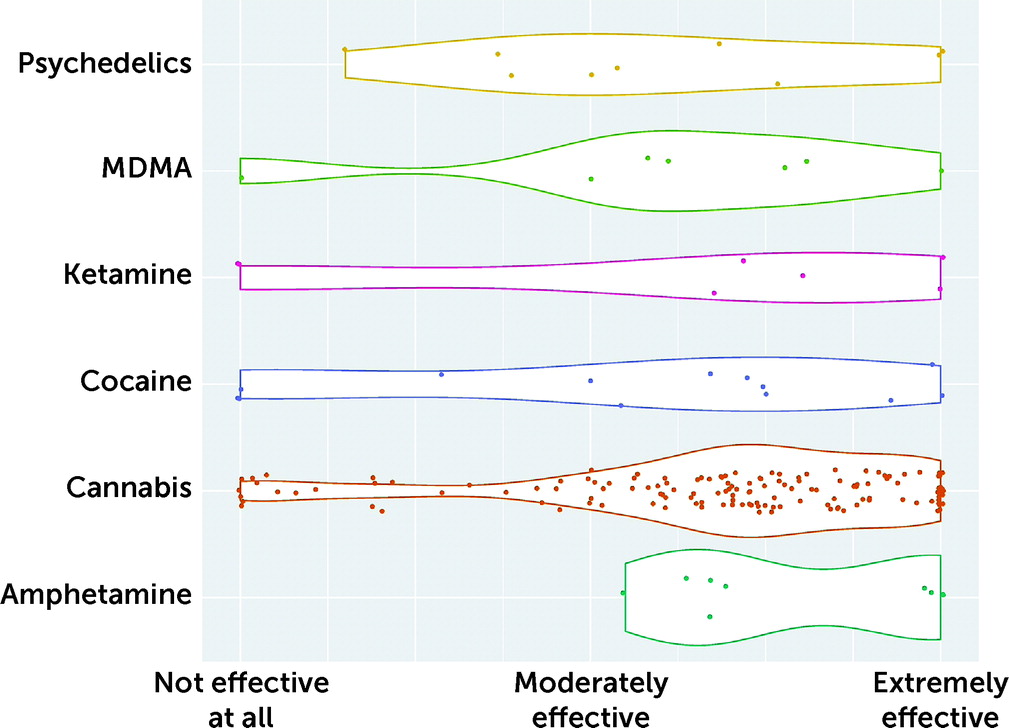
Respondents were asked to rate the effectiveness of each illicit substance on their FND symptoms. Median values (out of 100) were as follows: cannabis, 73.0 (N=142); ketamine, 72.0 (N=7); amphetamines, 69.0 (N=9); cocaine, 67.0 (N=13); MDMA, 61.0 (N=7); and psychedelics, 54.0 (N=10) (substances with use reported by <7 respondents were not included). The rated effectiveness of illicit substances is summarized in
Figure 2.
Willingness to consider medically supervised psychedelic therapy.
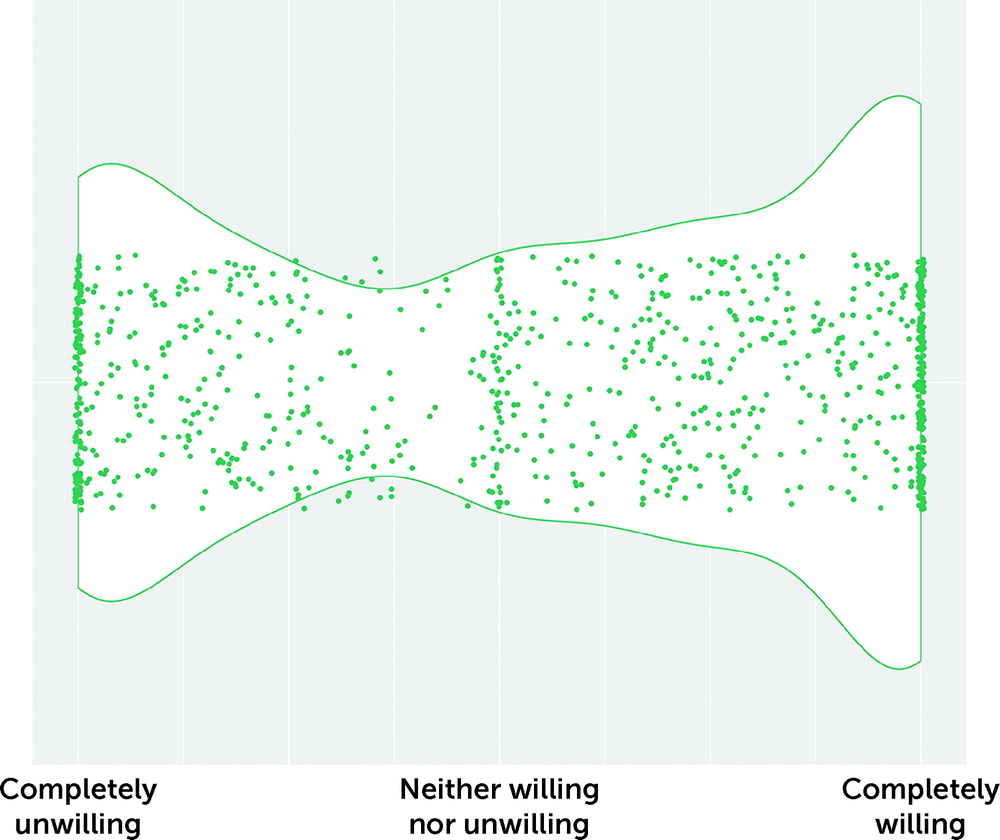
Respondents were asked whether they would be willing to consider using psychedelic substances in a medically controlled setting if they were shown to be safe and effective treatments for FND symptoms. Overall, 46% were willing (defined as a rating >67 on the visual analogue scale), whereas 35% were unwilling (defined as a rating <34 on the visual analogue scale). The remaining 19% were ambivalent (defined as a rating 34–67 on the visual analogue scale). Overall, there was a wide range in opinion, with a skew toward extreme responses and with a preponderance for a response of “completely willing” (
Figure 3).
Discussion
In this online study of persons with FND, we specifically sought to understand the prevalence of legal and illicit substance use as a self-management technique, the subjective effectiveness of these substances for FND symptoms, and views on novel medical therapies.
Legal Psychoactive Substances
Nearly half (46%) of the respondents indicated that they had used legal psychoactive substances to manage or cope with symptoms. They rated these substances on average to have a modest effect on FND symptoms. One previous study indicated that some patients with functional seizures use CBD for their symptoms (
29), with effects possibly modulated by its anxiolytic effects, which may itself be mediated by positive expectation (
30). To our knowledge, no other previous studies have examined the prevalence or efficacy of other legal psychoactive substances for management of FND symptoms.
In the present study, a small proportion of patients (8%) indicated that they had accessed prescription medication without a prescription. Patients may resort to “self-medication” due to factors such as an inability to access medication through a prescription or their own ideas about treatment that differ from that of health care professionals. There are recognized benefits of self-medication, including the active role of the patient in his or her own health care, although these are often outweighed by several drawbacks, including lack of monitoring, use of ineffective or harmful substances, issues with pharmacological interactions, and risk of dependence (
31).
Illicit Psychoactive Substances
In our survey, 15% of respondents reported having used illicit substances, primarily cannabis, for management of their symptoms. This survey was not designed to give an indication of the prevalence of recreational drug use among patients with FND, in part because we asked respondents to indicate only whether they had taken the substances to help alleviate their symptoms. Nevertheless, the proportion of respondents who had used illicit substances in this sample appears to be similar to, or slightly lower than, that in the general population (
32).
In our survey, the sample sizes were small, and, for most substances, there was a large variation in perceived effectiveness between respondents. We cannot draw any clear conclusions on the effectiveness of illicit substances based on these data. Additionally, our survey design was not equipped to address what specific effects the substances may have had on FND core and associated symptoms (e.g., substances may have had a more general positive effect on sleep and fatigue symptoms without directly affecting the core FND symptoms). Most respondents who used illicit substances reported either no or minimal physical and psychological sequelae, although a small number reported severe physical (N=5) and psychological (N=3) complications after taking cannabis, cocaine, or MDMA.
Effectiveness of psychoactive substances on symptoms is related to the specific pharmacological aspects of the agents (discussed below). Nevertheless, the placebo effect and the related phenomenon of expectation may also be common features relevant to the use of psychoactive substances in treating symptoms. The placebo effect is common to all illnesses, including neurological conditions such as Parkinson’s disease (
33). The placebo effect and expectation have been purported to be key contributors to response in recent trials of substances (including ketamine, MDMA, and psychedelics) used for neuropsychiatric conditions (
34). Patients with FND are often noted to have strong placebo responses mediated by the brain’s “placebo network,” which overlaps with regions implicated in the neurobiology of FND. The placebo response could be utilized synergistically when developing or administering treatments for FND (
33).
Cannabis.
Cannabis contains numerous psychoactive cannabinoids that act on the cannabinoid receptors in the CNS. It is believed that the endogenous cannabinoid system is involved in regulating pain responses (
35). In our study, 142 patients had tried cannabis to help control their symptoms, with on average a greater than moderate effectiveness and with a generally favorable side-effect profile. Physicians in the United Kingdom are legally permitted to prescribe cannabis compounds; however, prescribing rates remain low (
36). Cannabis products in the United Kingdom are currently approved for use in spasticity associated with multiple sclerosis, as well as for some rare childhood epilepsy syndromes (
37).
Literature on the use of cannabinoids for functional disorders is sparse; however, three patients with treatment-resistant functional tic disorder (a form of functional movement disorder) were described as showing “marked symptom improvement” with the use of cannabinoids (
38), and cannabinoids have been reported as being effective in managing symptoms in fibromyalgia (
39). It is possible that cannabinoids could offer some symptomatic relief to patients with FND, particularly if pain is a comorbid feature (which is common [
40]), and may therefore be of interest as an area for future research in FND.
Cocaine.
Cocaine is a stimulant and euphoriant substance that primarily exerts effects via inhibition of presynaptic dopamine reuptake (
41). In our study, 13 respondents indicated that cocaine had a moderate effect on their symptoms, although there was one report of both severe psychological and physical adverse effects. To our knowledge, there are no previous studies examining the effects of cocaine on any neuropsychiatric disorder, including FND. In addition, there are a number of factors that make examination of systemic cocaine administration challenging and risky, for example, the risk of acute adverse events, such as acute cardiovascular episodes (stroke and myocardial infarction), and risk of dependence (
42). The use of cocaine in medical settings is limited to topical administration in specialist otorhinolaryngological surgery (
43). Overall, cocaine does not appear to represent a good candidate for exploration in FND.
Amphetamines.
Amphetamines are stimulants (as well as euphoriants) that exert effects via dopamine and norepinephrine transporter inhibition (
44). Amphetamines and their derivatives are licensed in the United States and the United Kingdom for the treatment of attention-deficit hyperactivity disorder (
45,
46). In our study, nine respondents indicated that they had used amphetamines for their FND symptoms, with a reasonable degree of effectiveness. The single respondent who used mephedrone, an amphetamine-like stimulant, also reported extremely good efficacy; however, this was not the case with khat, which contains the stimulant cathinone.
In older studies, intravenous methylamphetamine administration has been described, with some success reported in case studies and series on FND from the 1950s to the 1970s (
47–
49). Very limited conclusions can be drawn from these studies, particularly because they used subjective and poorly defined outcome measures. It is, however, possible that medical amphetamines could offer a potential area of research for patients with some forms of FND, particularly if fatigue is a component (which is common [
28]), given their current license and safety record.
MDMA.
MDMA is a euphoriant and empathogenic substance that likely exerts its effects via serotonin release and reuptake inhibition (
50). In our study, the seven respondents who rated MDMA felt that it was moderately effective. To our knowledge, there has been only a single exploration of MDMA as a therapeutic substance specifically for FND. In a study published in 1976, seven patients were given doses of MDMA alongside psychological therapy, and significant reductions on scores measuring “hysterical tendencies” were observed, although results were not adequately reported, rendering further analyses unachievable (
51). More recently, MDMA has been shown to have potential efficacy in trauma-based psychopathologies such as PTSD. In these therapeutic paradigms, MDMA is administered alongside therapy. MDMA may additionally enhance the therapeutic alliance, a factor that often has a large impact on the outcome of psychotherapy (
26,
52,
53).
In our survey, one respondent reported severe psychological and physical adverse effects. Interpretation of this response is complicated by the fact that drugs sold as MDMA outside of clinical settings are likely to contain adulterants, many of which can lead to subjectively unpleasant experiences and can have (often harmful) side-effect profiles that are distinct from MDMA (
54). The likelihood of experiencing severe physical or psychological side effects is reduced in medically supervised settings, because the MDMA is manufactured to a medical standard. Indeed, the limited side-effect profile and early efficacy in trials of medically supervised MDMA therapy in other conditions such as PTSD means that MDMA, possibly in conjunction with psychotherapy, may warrant further exploration in neuropsychiatric conditions. This may be particularly the case in FND research, where a history of trauma is relevant for some (but not all) patients (
55).
Ketamine.
Ketamine is a dissociative anesthetic that acts as an uncompetitive
N-methyl-
d-aspartate receptor antagonist (
56). In our study, the seven patients who had tried ketamine felt that it was moderately effective. Ketamine has recently undergone clinical trials for neuropsychiatric conditions such as depression (
57). As with psychedelics (discussed below), the administration of a single dose of ketamine (or its S[+] enantiomer esketamine) can lead to lasting improvement in some patients with otherwise treatment-resistant depression (
58); repeated doses of ketamine have also shown promise in reducing symptom severity in patients with PTSD (
25). There have been two independent case reports of esketamine nasal spray used in patients with FND and refractory depression; in both cases, esketamine was prescribed for depression; however, both the mood disorder and FND improved (
21,
59).
The dissociative effects of ketamine may render its use in FND difficult; however, previous studies have indicated that the degree of response to ketamine infusion in depressed patients was positively associated with the degree of dissociation induced by the medication (
60). Additionally, other dissociative anaesthetics (such as propofol) have been administered in low doses in clinical settings to good effect in patients with FND. These administrations have been described as “therapeutic sedation” or “abreaction therapy” and share similarities with current protocols for medically supervised ketamine administration (
9,
61). Ketamine therapy may represent a further novel therapeutic avenue to research in FND, particularly in cases of motor FND and comorbid depression.
Psychedelics.
Classical psychedelics are partial serotonin 2A receptor agonists that have entheogenic and hallucinogenic properties (
23). In our study, 10 respondents had used psychedelics as a self-management technique. Self-reported physical side effects were minimal at worst, and many respondents reported that they would be willing to try medically supervised psychedelic therapy if it were found to be safe and effective, suggesting that this could be an acceptable novel treatment approach in this patient group. Placebo-controlled randomized controlled trials are currently under way to investigate psilocybin combined with psychological support in treatment-resistant depression (
62), and psychedelics are being investigated in other neurological and neuropsychiatric disorders at the brain-body interface, such as headache disorders (
14), anorexia (
63), chronic pain (
15), and PTSD (
64). Psychedelics also have research potential in alcohol use disorder (
65), tobacco addiction (
66), existential distress in life-threatening disease (
67), and obsessive-compulsive disorder (
68). Safety data from early-phase trials are reassuring (
17,
69), and psychedelic drugs are physiologically nontoxic and non-habit-forming in humans and animals (
62,
70,
71).
Some authors have suggested that psychedelic therapy may be an interesting area of exploration in FND (
17,
24), and there have been other suggestions that trials of psychedelic therapy in conjunction with physiotherapy or psychotherapy (current best practice) could be a future option (
22). Recently, several members of our research team summarized the “preprohibition literature” on psychedelic (“psycholytic”) therapy in FND, which indicated mostly favorable safety profiles and improvement in some patients, albeit in a small and heterogenous sample size (
23). In this context, we believe that research on this potential therapeutic avenue for FND is a justifiable consideration.
Strengths and Limitations
A more in-depth discussion of the biases, strengths, and limitations of the survey design is provided in the previous study from the same survey (
28). Briefly, the main strength of this study is the relatively large sample of respondents (>1,000), which increases the representative value of the responses. To our knowledge, this is the first large-scale survey of self-management techniques in persons with FND.
There were several limitations to this study. First, there may have been selection bias in patient participation toward those who were active online, and those well enough to complete a large survey were more likely to take part. Second, our survey required a good grasp of the English language, which may limit generalizability to non-English-speaking populations. Third, we did not ask respondents whether they felt that using these substances had been harmful or whether the substances had worsened their symptoms. Fourth, we did not ask respondents for information on medication or substance allergies or intolerances, which have been shown to be associated with illness duration (
72) as well as functional seizure diagnosis (
73), in previous samples. Fifth, we did not ask respondents about pain symptoms; this limited the analysis of some substances (e.g., cannabis) that are known to have pain management effects when used in this patient population.
In this survey, we relied on self-report of FND diagnosis, and participant answers were not objectively verified. However, we feel that our participant pool was unlikely to have featured significant numbers of non-FND patients, given that all respondents were required to indicate that they had been diagnosed with FND by a health care professional. Additionally, we feel that levels of “self-diagnosis” of FND are low, with a diagnosis often only reached after months or years of contact with health care services (
28).
Given the sensitive nature of the questions about illicit substances, some respondents may not have felt comfortable answering all of the questions in full, which may have led to an underestimation of illicit substance use. Despite this, around 93% of respondents who took the survey answered the questions on illicit substance use. Aside from cannabis, very few (N=1–15) respondents had used other illicit substances, and therefore data on the subjective effectiveness of these other substances (e.g., psychedelics, amphetamines, MDMA, and ketamine) should be interpreted with extreme caution. The survey was intended to be a descriptive account of self-management in FND, as opposed to a generalizable measure of effectiveness.
Conclusions
In this large international online study, we explored self-reported use of legal and illicit substances for self-management of FND symptoms. Our results indicate that a sizable minority of patients with FND attempt to manage their symptoms with both legal and illicit substances.
Previous data have indicated that novel pharmacological treatments that can be administered as single doses in medically supervised environments—such as amphetamines, ketamine, MDMA, and psychedelics—may have promise in several neuropsychiatric disorders. Given the current precedent of clinical trials utilizing substances such as ketamine, MDMA, and psilocybin, as well as early data suggesting efficacy and safety in other neuropsychiatric disorders, researchers could consider whether FND may represent a suitable target condition for research with these substances. Specifically, given the evidence of tolerability in older trials of FND, promising adverse-effect data in this survey, and reasonable patient acceptability indicated by these survey data, we suggest that a pilot trial of psilocybin for FND could be a justifiable option for future research.
Acknowledgments
The authors thank all the respondents to the survey. The authors also thank Dawn Golder and Bridget Mildon for their input in designing the survey, as well as the charities FND Hope, FND Action, and FND FrieNDs for assisting with the dissemination of the study survey.