Depressive symptoms are common in Parkinson’s disease (PD) and significantly affect patients’ quality of life.
1 The average prevalence of depression in PD was found to be approximately 40%.
2 A study conducted by the Global Parkinson’s Disease Survey Steering Committee showed that depressive symptoms accounted for 53% of the variance in scores on the 39-item Parkinson’s Disease Quality of Life Questionnaire, which was even greater than cardinal motor features.
3 However, the mechanism underlying depressive symptoms in PD has not yet been fully elucidated.
4Visual impairment and olfactory dysfunction have also been widely reported in patients with PD
5,6. In one study, about 78% of patients with PD reported at least one vision deficiency,
5 and the estimated prevalence of olfactory deficiency in PD was as high as 90%.
7 Previous research on major depressive disorder has suggested that the severity of depressive symptoms is correlated with the visual system in several aspects.
8–10 In addition, several studies reported that older individuals with visual impairment had a higher prevalence of depression compared with their peers with normal vision.
11,12 Over 60% of women with endogenous depression showed disturbed color vision in a previous study.
13 Meanwhile, therapeutic effects of light exposure to eyes were reported in several kinds of depression
14–18. These studies suggest that the visual system is crucial in the onset of depression. However, it is still unclear whether the occurrence of depressive symptoms in PD is related to visual impairment.
Olfaction was also reported to be linked with brain regions regulating emotion.
19 Some researchers have considered olfactory dysfunction to be an early indicator of various neuropsychiatric disorders.
20–22 However, the relationship between depressive symptoms and olfactory dysfunction remains controversial at present. In previous research, olfactory function was reported to be either reduced,
23,24 increased,
25,26 or unchanged
27–29 in patients with depression compared with healthy control subjects. There have been few studies of the relationship between olfactory deficits and depressive symptoms in patients with PD, and results have been inconsistent,
30–33 similar to research on patients with depression.
23–29 Thus, the association between depressive symptoms and olfaction in PD is still an open question that needs to be further investigated.
In the present study, we assessed the association between depressive symptoms and two sensory functions—color vision and olfactory function—in PD patients.
Methods
The study was approved by the Ethics Committee of Huashan Hospital. All participants provided informed consent for study participation.
Participants with PD were recruited from the clinic in the Department of Neurology at Huashan Hospital, Fudan University.All participants were without a past history of brain or eye surgery. Motor disability was evaluated by using the Unified Parkinson’s Disease Rating Scale (UPDRS-III) and staged by using the Hoehn and Yahr scale
34 during the “off” period, defined as being off antiparkinsonian medication at least 12 hours prior to study assessment.
Cognition was evaluated with the Mini-Mental State Examination (MMSE). Patients with an MMSE score below 24 were regarded as possibly being demented and were excluded from the study.
35 All participants were surveyed about their history of treatment with psychotropic medications, and individuals who had received such treatment within 6 months prior to the start of the study were excluded. For color vision testing, individuals who had the following issues were also excluded: 1) symptomatic cataracts, history of glaucoma, retinopathy, or other ocular diseases; and 2) ongoing treatment with medications that affect color vision, such as digitalis, antiepileptic drugs, and antituberculosis drugs.
Depressive symptoms were evaluated by using the Beck Depression Inventory-II (BDI-II)
36 and the 30-item Geriatric Depression Scale (GDS-30).
37 The BDI-II is a 21-item self-report assessment of depressive symptoms.
36 Previous studies have suggested that the BDI-II can be divided into several subdomains.
38–41 In the present study, scores on items 1–13 (mood, pessimism, sense of failure, lack of satisfaction, guilty feeling, sense of punishment, self-hate, self-accusations, suicidal ideas, crying spells, irritability, social withdrawal, indecisiveness) were summed to calculate the cognitive/affective score of BDI. Items 14–21 (worthlessness, work inhibition, sleep disturbance, fatigability, loss of appetite, weight loss, somatic preoccupation, loss of libido) were summed to calculate the somatic symptom score.
42The Farnsworth-Munsell 100 Hue Test (FMT) was used to measure color vision. In this assessment, participants were asked to arrange a series of colored disks in sequence between two reference disks, using binocular vision. The total error score (TES) on the FMT was calculated as described previously.
43 The room in which the test was performed was illuminated by a daylight lamp (Biolux, Osram, 6500K). The tests were conducted without time restrictions.
Olfactory function was assessed with the Sniffin’ Sticks Screening 12 Test.
44,45 In this assessment, pen-like sticks were placed approximately 2 cm in front of the participant’s nostrils. The participant then was asked to sniff the odorant with both nostrils for 4–5 seconds and select the odor smelled from among four options. Each odor presentation was undertaken no sooner than 20 seconds after the one preceding it. The number of correct odor identifications on the Sniffin’ Sticks Screening 12 Test made by each participant was used as his or her odor identification score, with scores ranging from 0 to 12.
Associations were assessed using Spearman’s rank correlation with an alpha set at 0.05. The manual backward deletion method was used to remove the independent variables that were not significant in the multiple linear analysis (p>0.05). In order to ensure that the correlations were not solely attributable to the effects of demographic variables and nondepressive symptoms in PD, all demographic and other explanatory clinical variables were added to the multiple linear regression model. All statistical analyses were conducted with R software, version 3.31 (The R Foundation, Vienna, Austria).
Discussion
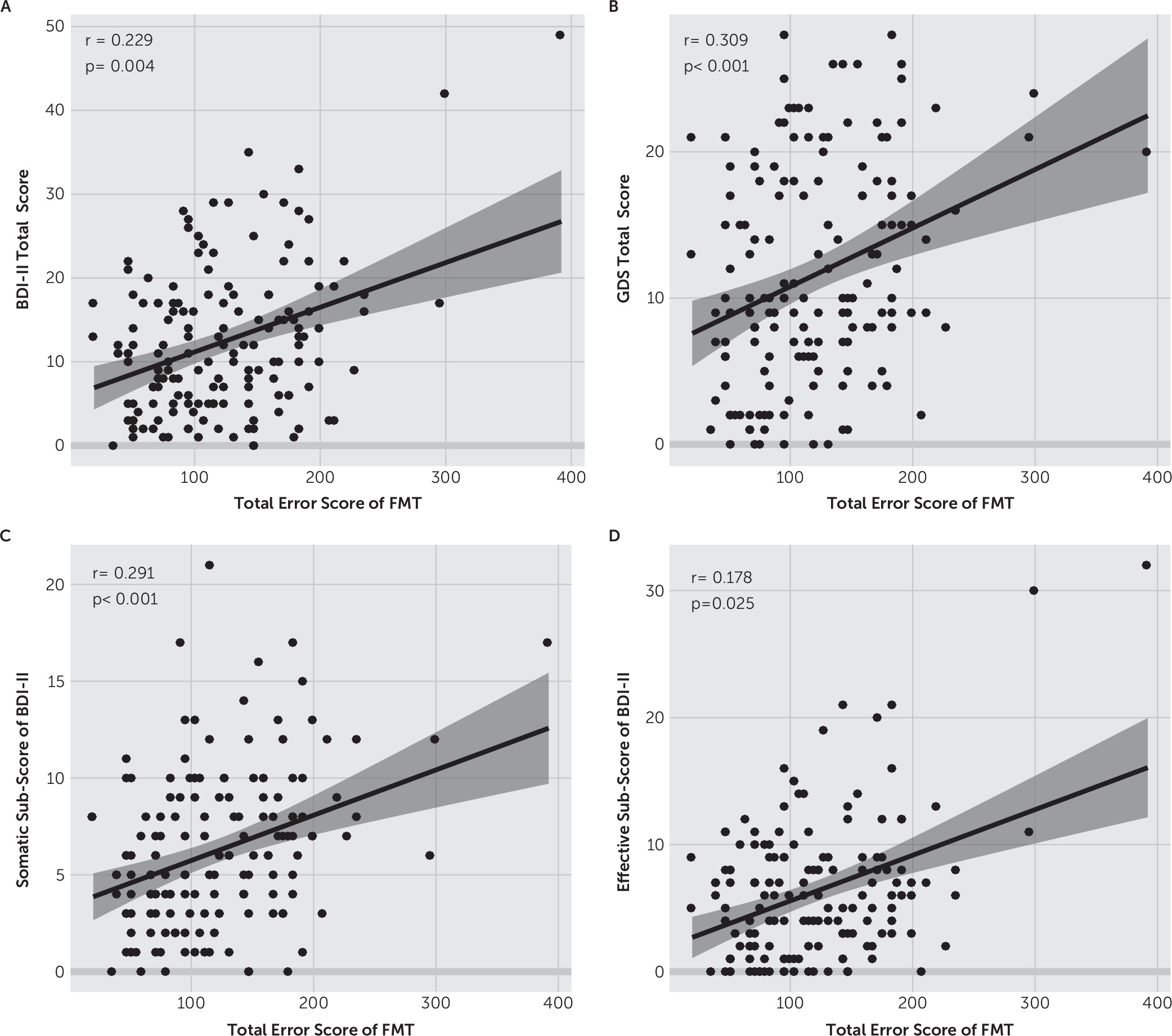
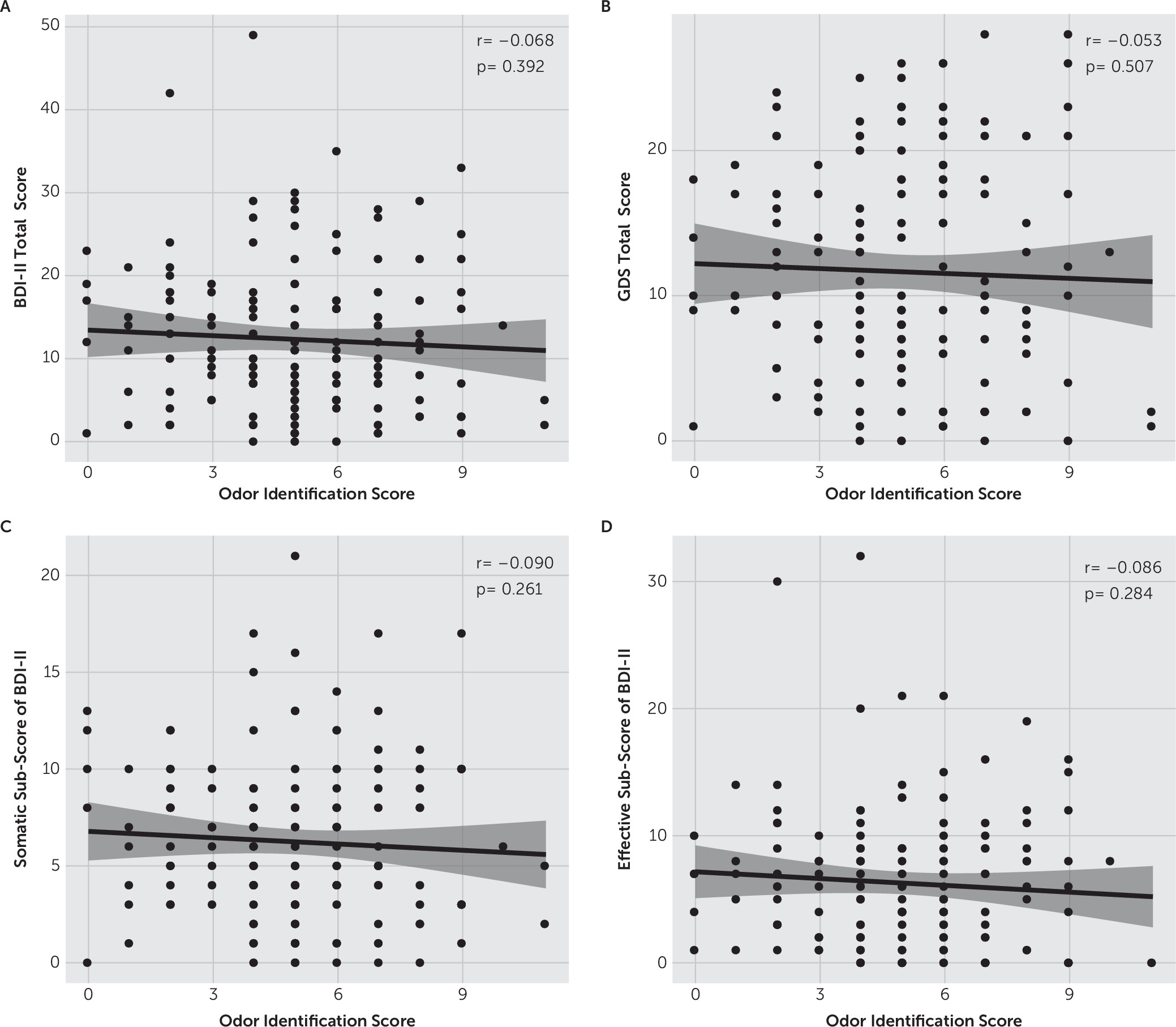
In the present study, we investigated whether there was an association between depressive symptoms and sensory dysfunction in PD patients. On the basis of our results, both the BDI-II and GDS-30 total scores were significantly correlated with the TES on the FMT in a positive manner. These results suggest that more severe depressive symptoms are correlated with poorer color vision in PD patients. However, there was no significant correlation observed between depressive symptoms and olfactory function measured with the odor discrimination test. To our knowledge, this is the first study to explore the association between depressive symptoms and visual function in PD patients. Our findings suggest that the decrease in color vision but not olfactory function is paralleled with the severity of depressive symptoms in PD patients, supporting the hypothesis that occurrences of depressive symptoms were linked with the vision system in PD.
Previous studies on major depressive disorder have suggested that dysfunction of the visual system is linked with depressive symptoms in several aspects. For example, Bubl and colleagues
49 reported a reduction in contrast discrimination performance and a decrease in pattern-electroretinogram-based contrast gain in patients with depression.
50 Another study showed disturbed color vision in 63% of women with depression.
13 In addition, several studies have reported visual impairment to be correlated with depressive symptoms in elderly individuals.
51–53 A longitudinal study of elderly adults also showed that visual dysfunction can be a strong predictor of both the onset and persistence of depression in older persons.
54Light therapy has been shown to be an effective treatment for depressed patients with PD
55,56 and depressive-related disorders.
14–18 One study showed that the antidepressive effect was much greater when light was applied to the eyes compared with the skin.
57 A 4-week study of light therapy found that light therapy could significantly increase visual contrast sensitivity in patients with seasonal affective disorder.
58 This evidence supports the idea that the visual system plays an important role in the development of depression in non-PD adults. Consistent with these results, our study provided evidence that the functional link between the visual system and depressive symptoms found in non-PD depressive disorders also existed in PD patients. Therefore, all these results may indicate that the occurrence of depressive symptoms in PD may share some etiologic mechanism with other depressive disorders that is potentially mediated by the visual system. There is a hypothesis that the occurrences of depressive symptoms may result from less sensitivity to environmental light,
59 which might be caused by abnormalities in the rods, cones, or other light-sensitive cells of the retina.
59,60 On the other hand, a recent study reported that atrophy of the occipital cortex was correlated with depressive symptoms in PD patients, suggesting that the visual cortex can also be an underlying site accounting for this association.
61 Further research will be needed to clarify this issue.
The results from previous studies of olfactory function in depressed psychiatric patients have been inconsistent. Some researchers have reported a decrease in olfactory performance in patients;
23,24 however, other researchers have reported that olfactory discrimination ability in depressed patients is unchanged,
27–29 or even increased.
25,26 This inconsistency also exists in the research on PD patients. One study reported that higher BDI scores were associated with worse performance on the University of Pennsylvania Smell Identification Test (UPSIT).
33 However, the presence of comorbid depression in PD did not affect the olfactory function examined with the Sniffin’ Sticks Screening 12 Test in a study conducted by Rossi and colleagues.
31 Another study using the UPSIT also suggested that there was no significant association between olfactory dysfunction and depressive symptoms in PD,
32 which is in line with the results of the present study.
There are some notable limitations of our study that need to be addressed. First, despite being evaluated by two depression scales, depressive symptoms were all self-rated, and the participants lacked a diagnosis for mood disorder. Second, examination of fundus and other visual functional tests, such as visual acuity, were not performed. Third, the cross-sectional design of our study renders it impossible to evaluate for a causal association between color vision and depression. Longitudinal data are needed to further understand the causal and temporal relations between loss of color vision and depression in PD.
In conclusion, our results demonstrated that depressive symptoms are associated with color vision but not olfactory function in PD. These results suggest that the visual system may play an be importantly related to the development of depressive symptoms in PD.