Amidst a strained U.S. mental health (MH) delivery system that is often inaccessible, inequitable, and poor quality (
1,
2), the coronavirus disease 2019 (COVID‐19) pandemic has placed children and adolescents (hereafter, children) at higher risk for new and worsening MH disorders (
3). The recent declaration of a national emergency in child MH (
4) and U.S. Surgeon General's Advisory on protecting youth MH (
5) underscores this crisis. During the first wave of the pandemic, nearly all of the 55 million kindergarten through 12th grade students in the U.S. were affected by school closures, along with 1.5 billion students around the globe (
3). The cumulative risk of loss of school‐based resources (
6), social distancing measures (
7), threats to financial stability and food security (
8,
9), parental unemployment (
10), substantial changes to daily routine and social infrastructure (
11) and exposure to high levels of stress and anxiety (
12), has been unsurprisingly associated with markers of worsening of children's MH (
6,
13) and placed an unprecedented burden on children and families, particularly families of color (
14,
15). One illustration of these impacts is in the findings of a national U.S. survey in June 2020, which found worsening MH for parents occurred alongside worsening MH for children in nearly 1 in 10 U.S. families, among whom 48% reported a loss in regular childcare, 16% experienced a change in insurance status and 11% reported worsening food security (
16). Increased domestic violence and child abuse hotline call volume at the onset of the pandemic suggested violence increased while children endured more exposure to perpetrators (
17). Despite concerns about increased MH needs of children, many social and school‐based services shut down during the early pandemic due to social distancing measures (
6,
18). These closures disproportionately affected families of children receiving special education resources (
19), marginalized youth (
15,
20), families affected by poverty (
21), and other populations with high child MH care need and low MH care access (
22).
In the aftermath of these still evolving threats to children's wellbeing, a better understanding who, when, and why children were brought to care for MH emergencies during the first wave of the COVID‐19 pandemic may help better identify youth vulnerable to the longitudinal impacts of the pandemic. Emergency departments (EDs) are often the first point of contact for children experiencing MH emergencies (
23). Throughout the pandemic, surveillance of MH‐related ED (MH‐ED) visits among pediatric populations during the COVID‐19 pandemic has provided a window into fluctuations in children's MH care use (
24). These efforts have demonstrated a disproportionate rise in MH‐ED use relative to ED use for other pediatric medical conditions: following national school closures in March 2020, child MH‐ED visits initially declined by 43% (
25), followed by a sharp proportional rise and persistent elevation relative to physical health conditions, observed through early 2021 (
24,
26). The proportion of child MH‐ED visits among children younger than 18 years old increased 66% during April 14–18, 2020 compared with April 14–21, 2019 (
25). During December 2020–January 2021, although ED visits overall were 25% lower than during the same months the year before, higher proportions of ED patients sought care for MH‐related concerns, particularly among children (
26). In addition to an expected rise in infectious disease related concerns, conditions with the highest proportional rise in of child ED visits during the pandemic included visits for open wounds to the limbs, suicidality (suicidal ideation, suicide attempts, and intentional self‐harm), schizophrenia spectrum and other psychotic disorders, feeding and eating disorders, and open wounds of the head and neck (
26).
Descriptive analyses of demographic trends during the COVID‐19 pandemic have suggested a higher proportional rise of MH‐related ED visits among females compared with males (
25), adolescents compared with younger children (
25), and among youth seeking care for suicidal ideation and attempts relative to other conditions (
27,
28). Others have raised concern that existing disparities in pediatric MH diagnosis and treatment have been accentuated, particularly among children vulnerable to loss of special education resources (
29). Despite progress in disentangling trends in MH‐ED use, there are several areas in which this early literature can be extended: first, given substantial co‐occurring demographic and diagnostic shifts in child MH‐ED use observed during the early pandemic (e.g., the simultaneous proportional rise in use by adolescent females and suicide‐related visits), analyses controlling for covariates (vs. descriptive unadjusted analyses) may further clarify subgroups uniquely vulnerable to increased service use or gaps in care (
25,
27,
30); second, pediatric‐focused studies reporting multivariate‐adjusted trends have predominantly occurred at single sites (
14,
24), and expansion to multi‐site analyses across geographically‐diverse regions of the U.S. may further capture national shifts in child MH‐ED use.
In this study, we build on recent studies by examining electronic health record (EHR) data from four geographically diverse regions of the U.S. to measure overall child MH‐ED use and variation in child MH‐ED use by diagnoses and child demographics (age group, sex, race, and ethnicity). Focusing on the early COVID‐19 pandemic (March–November 2020), corresponding to the initial onset of instructional disruptions and school closures, we compared the proportional change and adjusted risk for MH‐ED use prior to and during the COVID‐19 pandemic, matched by 36‐ and 12‐week intervals to capture relevant school impacts of the pandemic.
METHODS
Study Design and Data Source
This study is a retrospective, cross‐sectional cohort study comparing percent change and adjusted risk of child MH‐ED visits between 2019 (comparator) and 2020, matched on 36‐week (3/18/19–11/25/19 vs. 3/16/20–11/22/20) and 12‐week time intervals (spring: 3/18/19–6/9/19 vs. 3/16/20–6/7/20; summer: 6/10/19–9/1/19 vs. 6/8/20–8/30/20; fall: 9/2/19–11/25/19 vs. 9/1/20–11/22/20) to mitigate confounding by seasonal trends in child MH‐ED use. The index start date for 2020 corresponds to widespread classroom instructional disruptions and mandated school closures for 47 states (
31). The respective health systems' Institutional Review Boards deemed the study exempt. The need for participant consent was waived given the use of existing EHR data. The study followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
The data source was EHRs from four academic health systems spanning rural and urban U.S. regions (California, Florida, Massachusetts, and New York). Health systems were identified through the National Center for Advancing Translational Sciences (NCATS) Accrual to Clinical Trials (ACT) network. Requisite bidirectional Data Use Agreement were obtained to allow combination and analysis of aggregated and de‐identified EHR data.
Study Population
The study population included all ED visits (including those resulting in discharges, transfers, and hospitalizations) for children 3–17 years during the study time periods (March 18th, 2019, through November 25th, 2020, or March 16th, 2020, through November 22nd, 2020) and which were associated with a MH diagnosis in the same encounter. Presence of a MH diagnosis was defined by the occurrence of any of the following International Classification of Diseases Version 10 (ICD‐10) codes in any diagnostic field: F01‐F99.x, R45‐R46.x, T14.91x, X71‐83x. We excluded children younger than 3 and youth 18 and older to focus on children likely affected by school closures.
Study Variables
The main dependent variable was change in MH‐ED use defined by the number of MH‐ED visits by unique children occurring within each 36‐ or 12‐week interval. The main independent variables were child sociodemographics, diagnoses, and study site. Demographic characteristics were age group (young children: 3–5 years old, school‐age children: 6–12 years old, and adolescents: 13–17 years old), sex (male, female), race (due to low sample size for certain groups, analyses were conducted comparing Asian, Black or African American, White, and a combined Other/missing group), and ethnicity (Hispanic and non‐Hispanic). Mental health diagnostic groups were categorized by ICD‐10 codes to yield 12 diagnostic groups and suicide or self‐harm. For each child, a diagnosis was considered present if recorded in any field (primary, secondary etc.).
Analysis
Unadjusted analysis was performed by descriptive comparison of percent volume change in MH‐ED visits at each 36‐ and 12‐week time period. Significance testing for unadjusted analyses was performed by approximating the standardized normal deviate. Adjusted incidence rate ratios (IRR) were calculated using multivariate Poisson regression including sex, race, ethnicity, age group, site, and ICD‐10 MH diagnostic code category as covariates. Adjusted IRR were calculated by exponentiating the regression coefficients to estimate the ratio by which the dependent variable changed in for a unit change in each independent variable. To generate cluster‐robust inference, regression models were run with vce(robust) option to obtain robust error estimates for parameter estimates (
32). The ICD code category group F40–49 (anxiety disorders) was selected as the reference category for interpretability as this was the largest group corresponding to a circumscribed subset of diagnoses. To account for nonindependence among predictors, frequency of each predictor combination was reconstructed based on aggregated counts and individual‐level data.
p‐values less than 0.05 were considered statistically significant. All analyses were performed in Stata, version 17 (StataCorp).
RESULTS
Unadjusted Analyses
Compared with spring‐fall 2019, MH‐ED visits were reduced during spring‐fall 2020 (
n = 3892 vs.
n = 5228, −25.5%) and during each season: spring (
n = 1051 vs.
n = 1839, −42.8%), summer (
n = 1430 vs.
n = 1469, −2.6%), and fall (
n = 1411 vs.
n = 1920, −26.5%;
Table 1).
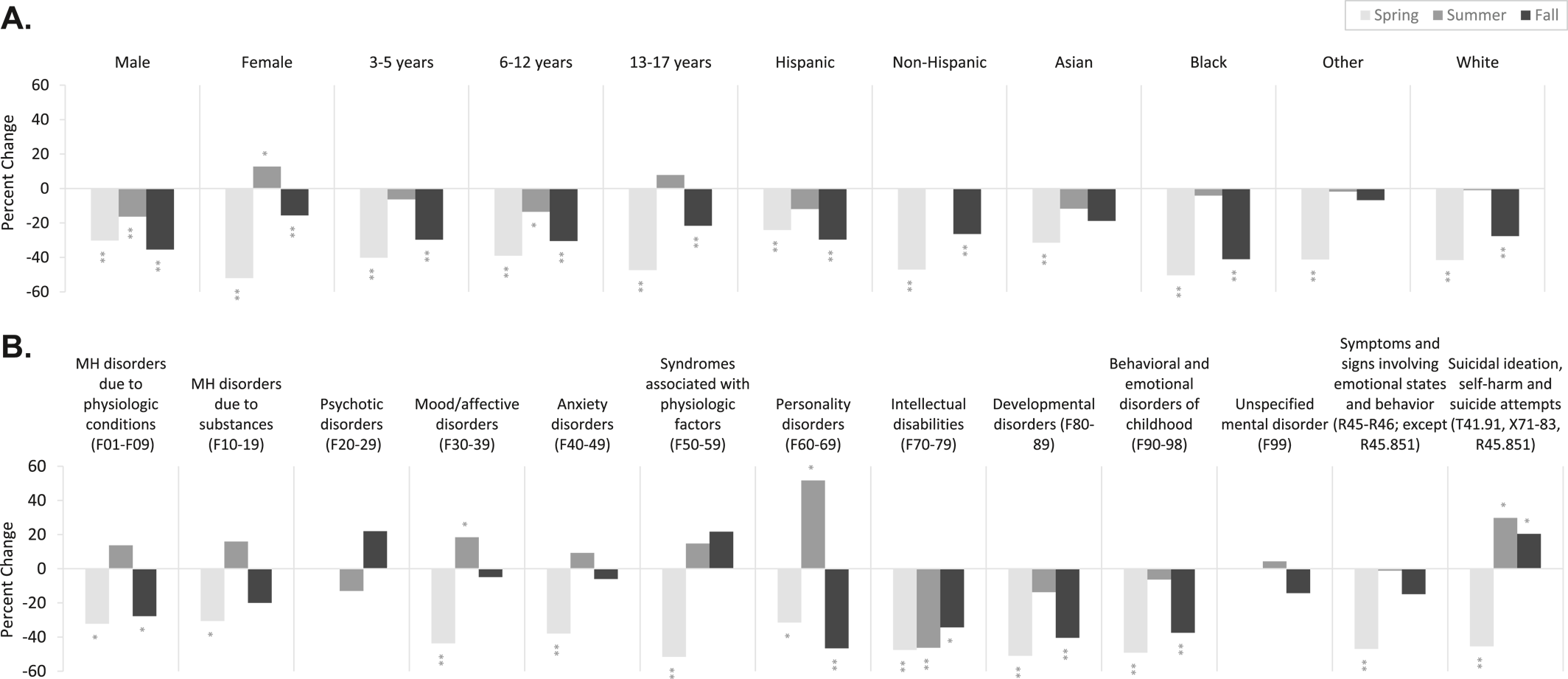
Compared with spring‐fall 2019, pediatric MH‐ED visits were most reduced among males versus females (−28.2 vs. −22.9%), school‐age children (−28.6%) versus young children (−25.9%) and adolescents (−22.9%), non‐Hispanic youth versus Hispanic youth (−26.6% vs. −22.3%), and Black youth (−34.8%) versus youth of other races (−17.7% to −24.9%;
Figure 1A).
Examination of demographic trends by season revealed that both males and females experienced a reduction in MH‐ED visits in spring, with greater proportional reduction among females (−52.5% vs. −30.3%). In summer, visits among females exceed males with a rise in MH‐ED visits compared with 2019 (+12.7% vs. −16.3%). In fall, both sexes experienced a reduction in visits relative to 2019, with greater proportional reduction among males (−28.2% vs. −22.9%). In spring, all age groups experienced a reduction in MH‐ED visits, with the greatest proportional decline among adolescents (−47.3%) compared with young (−40.1%) and school‐age children (−39.0%). In summer, visits among adolescents rose relative to 2019 (+7.8%). In fall, the greatest proportional decline was observed among young (−29.7%) and school‐age children (−30.5%) compared with adolescents (−21.6%). In spring, non‐Hispanic (−47.0%) and Black (−50.4%) youth experienced the greatest proportional reduction in visits compared with Hispanic (−24.1%) youth and youth of other races (−31.5% to −41.2%). In summer, Hispanic (−11.7%) and Asian (−11.7%) youth experienced the greatest proportional reduction in visits compared with other races (−4.1% to +0.4%). In fall, Hispanic (−29.6%) and Black (−41.1%) youth experienced the greatest proportional reduction in visits compared with youth of other races (−27.6%–6.8%).
Compared with spring‐fall 2019, MH‐ED visits were most reduced among youth with pervasive and specific developmental disorders (F80–89) (−17.0%), childhood‐onset disorders (e.g., attention deficit and hyperactivity disorders) (F90–98) (−18.0%), and symptoms and signs involving emotional state, appearance, and behavior (R45‐R46, except suicidal ideation R45.851; −17.7%;
Figure 1B). Mental health‐related emergency department visits rose absolutely compared with 2019 for unspecified mental disorders (F99) (+6.9%).
Examination of diagnostic trends by season revealed a proportional reduction in all MH diagnoses in spring, particularly among behavioral syndromes associated with physiological disturbances and physical factors (F50–59) (−51.6%), developmental disorders (F80–89) (−51.0%), and childhood‐onset disorders (F90–98) (−49.1%). In spring, pediatric MH‐ED visits rose absolutely compared with 2019 for unspecified mental disorders (F99) (+23.8%). In summer, there was variation amongst diagnoses, with greatest proportional rises suicide‐related diagnoses (T14.91, X71‐83, R45.851; +29.8%), and mood/affective disorders (F60–69; +51.7%), and greatest reductions in developmental disorders (F80–89; −13.8%). In fall, there was a sustained proportional rise in visits for suicide‐related diagnoses (+20.4%) and a sustained proportional reduction in visits for developmental disorders (F80–89; −40.4%).
Adjusted Analyses
In covariate‐adjusted analyses, compared with spring‐fall 2019, the adjusted risk for MH‐ED use in spring‐fall 2020 was lowest for school‐age children (IRR 0.83 [0.69–0.99]) compared with young children and adolescents (
Table 2). There were no other significant differences by sex, age group, race, or ethnicity. In contrast, in covariate‐adjusted analyses by season, relative to spring 2019, in spring 2020, the adjusted risk for MH‐ED use was greater for males (IRR 1.43 [1.11–1.87]) and Hispanic youth (IRR 1.36 [1.07–1.73]), but lower for young (IRR 0.22 [0.18–0.26]) and school‐age children (IRR 0.78 [0.63–0.97]), compared with females, non‐Hispanics, and adolescents, respectively. Relative to summer 2019, in summer 2020 there were no significant differences in risk by sex, age group, race, or ethnicity. Relative to fall 2019, in fall 2020 the adjusted risk for MH‐ED use was lower for males (IRR 0.73 [0.55–0.95]) compared to females.
Relative to spring‐fall 2019, the adjusted risk for MH‐ED use during spring‐fall 2020 was significantly lower for children with intellectual disabilities (IRR 0.62 [95% CI 0.47–0.86]), developmental disorders (IRR 0.71 [0.54–0.92]), and childhood‐onset disorders (IRR 0.74 [0.56–0.97];
Table 3). Significant changes in diagnostic groups were also found by seasons. In covariate‐adjusted analyses, in spring, MH‐ED use was significantly lower among children with developmental disorders (IRR 0.57 [0.37–0.88]) and childhood‐onset disorders (IRR 0.61 [0.38–0.98]). In summer, MH‐ED use was significantly lower among children with intellectual disabilities (IRR 0.41 [0.26–0.63]) and in fall, MH‐ED use was again significantly reduced among children with developmental disorders (IRR 0.57 [0.36–0.87]) and childhood‐onset disorders (IRR 0.61 [0.38–0.98]). Of note, the fluctuation in other diagnostic groups observed in univariate unadjusted comparisons (including proportional rise in suicide‐related visits), was not statistically significant when adjusting for covariates.
DISCUSSION
This study measured changes in MH‐related ED use among U.S. children aged 3–17 years, using EHR data from four academic health systems from March 1 through November 22, 2020, compared with a similar time period in 2019. Overall, pediatric MH‐ED use substantially declined in spring‐fall 2020, compared to the same period in 2019, with variation by season, sex, race, ethnicity, age group, and diagnosis. Consistent with other studies examining variation in child MH‐ED use during the early pandemic, we observed an initial decline in use in spring 2020, followed by a rise during summer 2020 and an attenuated reduction in fall 2020.
Unadjusted analyses paralleled other studies examining demographic trends by sex (
28), finding a greater proportional decline among females in spring followed by a proportional and sustained rise in visits among females in summer and fall. Similarly, our findings of variation in MH‐ED use by age group lend further support to a greater proportional decline among adolescents in spring followed by a proportional and sustained rise in visits by adolescents compared with younger age groups in summer and fall (
33). In parallel, when examining univariate trends in MH‐ED use by diagnoses, our findings were consistent with other work suggesting an initial decline in suicide‐related visits and mood, followed by a proportional and sustained rise in suicide‐related visits in summer and fall 2020 (
27,
28). Univariate analyses further suggested a disproportionate reduction in MH‐ED use in spring‐fall 2020 among Black youth compared with other races (35% vs. 18–25%), paralleling calls to action to address gaps in MH service access (
15).
Nevertheless, the simultaneous proportional rise in visits among females, adolescents, and for suicide‐related diagnoses during summer and fall 2020, and proportional reduction in visits among males, younger children, and children with developmental disorders and intellectual disabilities underscores the importance of adjusting for demographic and diagnostic covariates and the limitations of univariate comparisons in examining these trends (
30). In fact, when adjusting for demographic covariates using multivariate regression, differences in frequency of many diagnoses did not reach statistical significance. In adjusted analyses, we discovered a sustained and statistically significant proportional reduction in visits among children with intellectual disabilities, developmental disorders, and childhood‐onset disorders. There was no significant difference in proportional change between males and females or across races or ethnicities, though school‐age children experienced a significant decline in visits throughout spring‐fall compared with other age groups. This may suggest that univariate comparisons may tell an incomplete story, as demographic shifts (e.g., increased visits among females, adolescents) may explain observed shifts in diagnoses (e.g., increased visits for suicide‐related conditions) and vice versa.
Relative to other conditions, the adjusted risk for MH‐ED use was lower for children with intellectual disabilities, developmental disorders, and childhood‐onset MH disorders. Reduction in MH‐ED use in this population was statistically significant in spring‐fall 2020 compared with 2019. One reason for decline in MH‐ED use could be that these children are potentially more vulnerable to missed detection in the context of loss of full‐time in‐person instruction and disruptions to special education resources (
34). A growing literature has identified that the pandemic has had disproportionately negative effects on the ability of individuals with developmental disorders and intellectual disabilities to receive health care and daily support that they typically receive (
35,
36). It is also possible that hospital avoidance during the early pandemic explains this trend: individuals with intellectual disabilities are more likely to be admitted to the hospital for COVID‐19 and more likely to experience mortality (2.75–8x higher than the general population) due to COVID‐19 (
34,
37,
38).
There are several limitations to this study. This is a retrospective cross‐sectional analysis of naturalistically collected EHR data. Our analysis purposefully focuses on the early pandemic period corresponding to national school closures and findings may not generalize to resumption of in‐person or hybrid schooling. Nevertheless, our focus may shed light into the acute effects of the early pandemic during 8 months of an unprecedented stressor affecting nearly all U.S. children. We also used aggregated EHR data, thus children with repeated visits across varying 12‐week time periods may be counted more than once. The data have an error margin of +/−3 individuals. It was also not possible to distinguish between primary and secondary diagnoses, thus children may have presented for primary physical health concerns with a secondary MH diagnosis. Due to limitations in accessing multi‐site EHR data, we used an abbreviated list of ICD codes corresponding to convenience categories (e.g., F01–F09, F10–F19), and future work could stratify diagnoses in a more nuanced way by using other standardized classifications of MH disorder codes for children such as the class Child and Adolescent MH Disorders Classification System (
39). Finally, suicide‐related visits are likely incompletely captured using ICD‐10 codes, with some estimates suggesting as few as 3% of suicide‐related visits receive a corresponding ICD code (
40,
41). Further studies should explore use of both structured (e.g., chief complaint, problem list, triage screening scores) and unstructured (e.g., clinical text) data to identify these visits more accurately.
Overall, our findings suggest that early pandemic‐associated shifts in use of emergency MH services among children were highly variable, differentially impacting children and families particularly vulnerable to loss of school‐based resources. Going forward, areas of future research include longitudinal examination of COVID‐19‐related hospital avoidance, missed detection of MH diagnoses in the context of instructional disruptions, and associations with social determinants of health as well as other indicators of child wellbeing.
ACKNOWLEDGEMENT
The authors would like to thank Yujun Chen, M.S. and the integrated data repository team (University of Florida) and Karen Lopez, B.S. (University of California, Los Angeles) for their consultation on electronic health record data extraction. This study was funded in part by an American Psychiatric Association Foundation Small Research Grant and the National Center for Advancing Translational Sciences (NCATS) UL1TR001881 and UL1TR001427. JE is supported by the National Institute of MH (NIMH) training grant T32MH073517‐12.