There has been an interest over the past 50 years in the association between chronic latent parasitic infections, such as toxoplasmosis, and mental health diseases (
1).
Toxoplasma gondii (
T.gondii) is an ubiquitous intracellular neurotropic parasite, thought to infect about one third of the entire human population (
2,
3). In the brain,
T.gondii forms intracellular cysts within neurons, establishing chronic infection (
4,
5). Animal models show that
T.gondii infected mice exhibited behavioral changes, causing fatal attraction to feline predators (
6,
7,
8,
9). Moreover, it is thought that exposure to
T.gondii causes significant brain and behavioral anomalies in humans (
10). Furthermore, latent
Toxoplasma infection in the brain has been associated with widespread brain immune activation, increased blood brain barrier permeability, neural disruption, increased dopamine release in dopaminergic neurons (
11) and N‐methyl‐d‐aspartate receptor (NMDA) dysfunction (
12,
13). Yet, there is heterogeneity in the results across these studies (
14).
Toxoplasma infection appears to be a risk factor independent of the known genetic risk factors for schizophrenia (SCZ) (
15). The global age‐adjusted SCZ prevalence in 2016 was estimated to be 2.8/1000 persons (
16) and globally, SCZ cases rose from 13.1 million in 1990 to 20.9 million in 2016 (
16,
17). Treatment with medications that have anti‐toxoplasma activity (TATA) (
18), such as haloperidol and valproate, have been reported to be associated with lower rates of certain SCZ phenotypes (
12).
METHODS
Search strategy
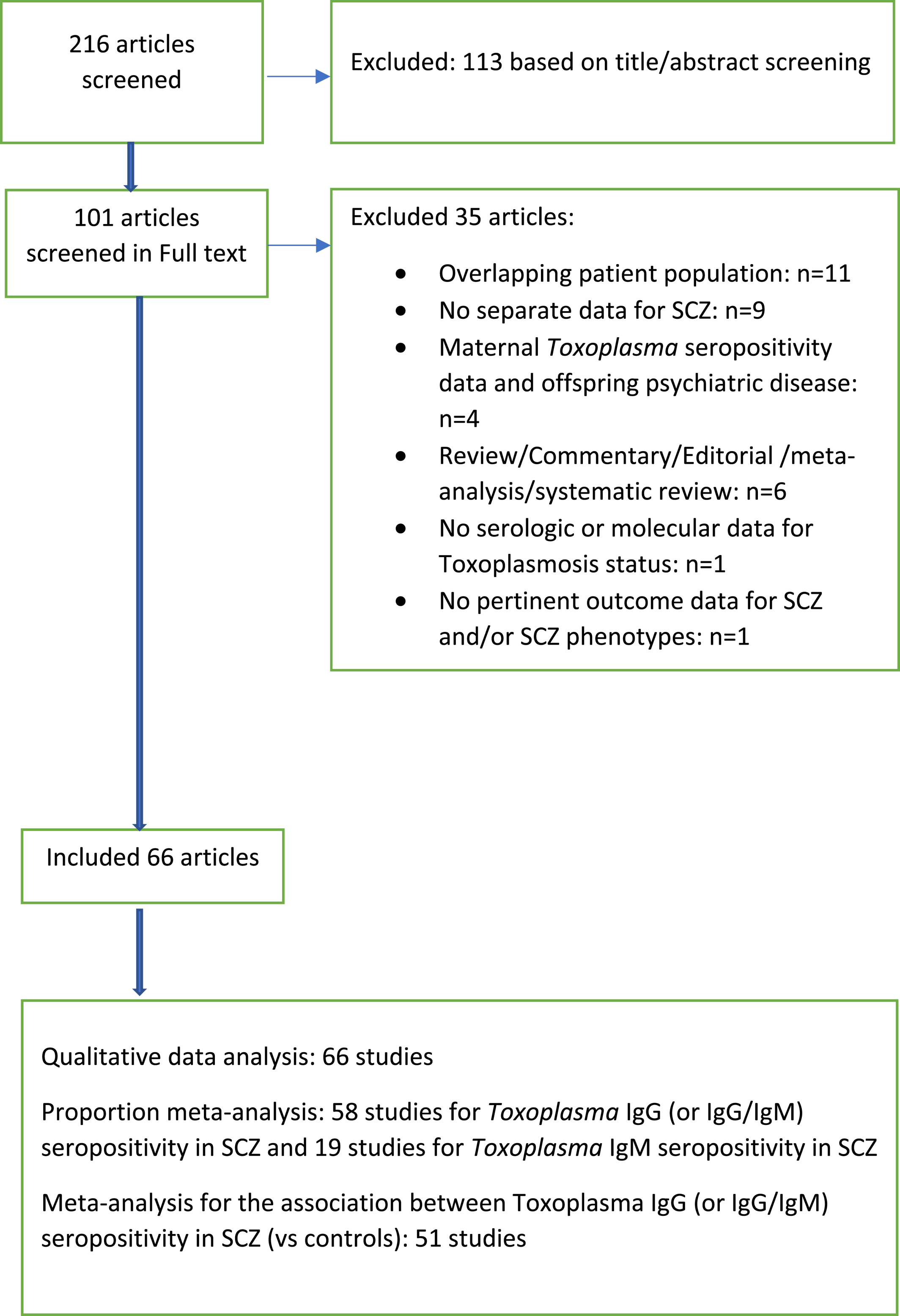
We considered studies included in previous meta‐analyses (
19,
21,
24,
25,
26) and performed updated PubMed searches to bring the database up to date (last search February 2021;
Figure 1). Our search strategy is listed in Appendix
1. We also perused the reference lists of key review papers for the identification of additional pertinent studies. Articles were screened for eligibility by two independent reviewers (Maria Gianniki, Despina G. Contopoulos‐Ioannidis).
Inclusion and exclusion criteria
We included studies of any type of study design (case control studies, SCZ cohort studies and population cohorts) that reported the association between toxoplasmosis and SCZ and/or specific SCZ phenotypes. We kept the definitions for toxoplasmosis and SCZ used by the study authors. The
Toxoplasma infection status could have been ascertained by serologic qualitative methods (
Toxoplasma IgG or IgM seropositivity), serologic quantitative methods (
Toxoplasma IgG or IgM titers/serointensity), or molecular methods. When more than one study existed from the same team with overlapping patients for the same type of SCZ outcome (SCZ or SCZ phenotype), we kept only the publication with the largest number of patients with SCZ. Studies exploring the association between maternal
Toxoplasma seropositivity and SCZ in the offspring (
27,
28,
29,
30,
31,
32,
33,
34) were not included in our analysis. Positive
Toxoplasma IgG antibodies in the newborn infant's Guthrie card blood sample reflect the maternal
T. gondii infection status from transplacentally transferred maternal
Toxoplasma IgG antibodies to the fetus, and additional neonatal testing is required to confirm whether the newborn infant is congenitally infected or not. We excluded reviews, commentaries and editorials with no original data.
Data extraction
From each eligible study we extracted the following information: author, year of publication, country of patients with SCZ, chronologic period of SCZ patients follow‐up, study design, diagnostic method for ascertainment of toxoplasmosis infection status, type of SCZ outcome targeted (e.g., SCZ and/or specific SCZ phenotypes), study sample size, N of patients with SCZ, N of controls analyzed, N of patients with additional mental health conditions targeted, inclusion criteria for patients with SCZ, types of selected controls (e.g., healthy volunteers, relatives of patients, random sample of patients from other hospital wards, etc.), types of adjustments or matching for confounders between cases and controls, adequacy of adjustments (e.g., adjustments for at least age and socioeconomic status/place of residence), and addressing of temporality (e.g., toxoplasmosis diagnosed before the diagnosis of SCZ).
We considered the following types of toxoplasmosis exposures: Toxoplasma IgG (or IgG/IgM) seropositivity, Toxoplasma IgG serointensity (Toxoplasma IgG titers analyzed as a continuous, categorical or binary variable), Toxoplasma IgM seropositivity, Toxoplasma IgM serointensity (Toxoplasma IgM titers analyzed as a continuous or categorical variable) or Toxoplasma polymerase chain reaction [PCR].
Moreover, we considered the following types of SCZ outcomes: SCZ (any type) or specific SCZ phenotypes (according to age at onset, duration of illness, total SCZ symptom score, positive and negative symptom scores, patient's awareness of illness, patient's compliance with medications, specific SCZ course [e.g., first episode, single episode with remission, single episode with partial remission, episodic without residual symptoms, episodic with residual symptoms, continuous], specific SCZ status [e.g., chronic, partially cured, cured], and types of SCZ [e.g., catatonic, disorganized, paranoid, residual, undifferentiated]).
Data extraction from each study was done by two independent investigators (Maria Gianniki/Angeline Ai‐Nhi Truong and Despina G. Contopoulos‐Ioannidis); discrepancies were reached by consensus.
Qualitative data analysis
We analyzed the prevalence of Toxoplasma IgG (or IgG/IgM combined) seropositivity rate in SCZ and in SCZ phenotypes thereof, the prevalence of Toxoplasma IgM seropositivity in SCZ and SCZ phenotypes thereof, the prevalence of Toxoplasma PCR positivity in SCZ and SCZ phenotypes thereof, and the prevalence of Toxoplasma IgG (or IgG/IgM) seropositivity in controls (if applicable). We analyzed the reported associations (and statistical significance thereof along with 95% confidence intervals [CI]) between toxoplasmosis and SCZ (and/or SCZ phenotypes). Moreover, we created a compilation list of reported claims and pathophysiologic theories to support the biologic plausibility of these associations.
Quantitative data analyses
We used descriptive statistics to analyze the study characteristics. We generated a world‐map of the countries of patients with SCZ.
Quantitative data were synthesized by proportion and association meta‐analyses. Proportion meta‐analyses were also used as not all studies had a control group to allow for the calculation of an association effect size. Proportion meta‐analyses synthesized across studies the
Toxoplasma IgG (or IgG/M) and IgM seropositivity rates in patients with SCZ (and in controls respectively) and calculated an average
Toxoplasma IgG (or IgG/IgM) and IgM seropositivity rate in these two groups (and 95% CI thereof) across studies. Association meta‐analyses synthesized across studies the effect sizes for the association of toxoplasmosis in SCZ versus controls, and calculated a summary odds ratio (OR) of
Toxoplasma IgG (or IgG/IgM) seropositivity in SCZ and 95% CI thereof. When both adjusted and unadjusted effect sizes or raw data were reported, we always preferred the adjusted effect sizes over other metrics. The data across studies were synthesized using the DerSimonian and Laird random effects model (REM) method (
35). Studies in these models were weighted by the inverse of their variance (
35). The heterogeneity across studies was calculated using the
I2 metric (
36).
I2 > 75% was considered a large heterogeneity. When there is large heterogeneity across studies in a meta‐analysis, the average estimates may be misleading; in those cases, reporting of the median and interquartile range (IQR) of the respective estimates across studies may be more informative. The Egger's test of bias (
37) was applied to test for small study effect bias; the Begg's funnel plot was also created.
Predefined subgroup association meta‐analyses were performed according to study adjustment status between SCZ and controls (studies with and without adjustment for important confounders [e.g., age, socioeconomic status/place of residence]), according to the assessment of the temporality of Toxoplasma IgG seropositivity in relation to the time of SCZ diagnosis, and according to study design (population cohorts or case control studies).
We used meta‐regression analyses to analyze across studies the association between the effect size (logarithm of the OR [logOR] of the association between
Toxoplasma IgG [or IgG/IgM] seropositivity and SCZ) and the control group
Toxoplasma IgG (or IgG/IgM) seroprevalence. The
metareg STATA command was used. Moreover, we used multivariate regression analyses to explore the impact of different factors (adjustment for confounders; assessment of temporality, study design or sample size) in the reported effect size of the association. The results of the meta‐analysis were reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (
38) (Appendix
2).
Statistical analyses
Analyses were performed in STATA software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). The Comprehensive Meta‐analysis software (Comprehensive Meta‐Analysis Version 3 Borenstein, M., Hedges, L., Higgins, J., & Rothstein, H. Biostat, Englewood, NJ 2013) was also used to compile effect size data reported in diverse formats across studies (raw 2 × 2 data, OR, risk ratio [RR] or hazard ratio [HR]).
DISCUSSION
We analyzed 66 studies published over the past 2 decades with 11,540 patients with SCZ and 69,491 controls, exploring the association between toxoplasmosis and SCZ or SCZ phenotypes. This large accumulated research agenda reflects the great interest of the scientific community for the identification of potentially preventable and/or treatable risk factors for SCZ (
40). Although there was large heterogeneity across studies in the types of toxoplasmosis exposures and SCZ outcomes targeted, on average, 45% of patients with SCZ were
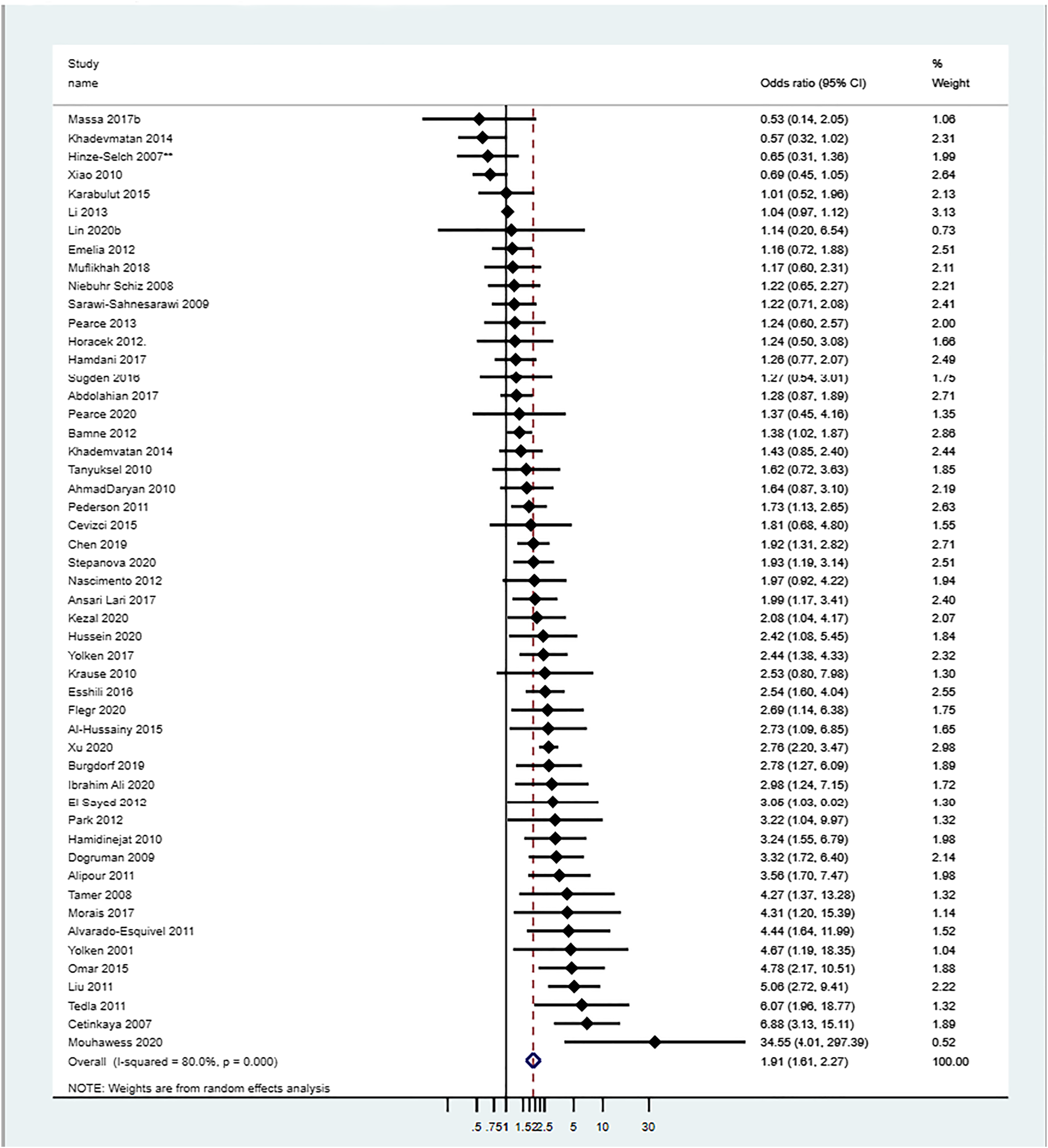
Toxoplasma IgG (or IgG/IgM) seropositive versus 30% of controls.
Toxoplasma IgG (or IgG/IgM) seropositivity increased the odds of SCZ by 1.91‐fold. This is similar to the estimate from an earlier meta‐analysis by Sutterland et al. in 2015 (OR = 1.81; 95% CI 1.52–2.17) (
19).
Most studies targeted only
Toxoplasma IgG (or IgG/IgM) seropositivity in SCZ. In contrast, the association with specific SCZ phenotypes was targeted in less than half of the studies. A positive association between
Toxoplasma IgG (or IgG/IgM) seropositivity and at least one SCZ phenotype was reported in only a third of the analyzed studies. We did not perform a meta‐analysis for the association between toxoplasmosis and SCZ phenotypes as a meta‐analysis for this association was recently published by Sutterland et al. (
25) in 2020. In this meta‐analysis no overall association was seen between
Toxoplasma IgG seropositivity and severity of total, positive or negative SCZ symptoms (
25). A significant association was only detected in the subgroup of patients with SCZ with a shorter duration of illness (less than 10 years), with
Toxoplasma IgG seropositivity being associated with more severe positive symptoms (
25).
We identified important methodological biases in the analyzed studies. Approximately 75% of the studies did not perform adjustments for important confounders such as age and socioeconomic status, or place of residence. Moreover, only four studies (
41,
42,
43,
44) had addressed whether the
Toxoplasma infection preceded the diagnosis of SCZ. In the absence of temporality assessment, causality is uncertain.
Toxoplasma infections may be the cause or the result of SCZ (e.g., due to common environmental exposures during hospitalization of patients with SCZ). Three out of 4 studies properly addressing temporality documented a positive association between
Toxoplasma infection and SCZ. Burgdorf et al. 2019, a large prospective cohort study in Denmark, showed increased rates of
Toxoplasma infection preceding the diagnosis of SCZ (
41). Another prospective population cohort study from Denmark by Pedersen et al. of 45,609 women also showed that high
Toxoplasma IgG levels before delivery were associated with a significantly increased risk of developing SCZ spectrum disorders subsequently (
44). Furthermore, a study among US military personnel in whom
Toxoplasma IgG levels were obtained before the diagnosis of SCZ also showed an association between higher
Toxoplasma IgG levels and SCZ (
43).
Schizophrenia has traditionally been assumed to be largely a genetic condition, with high heritability (
45). However, there is concern that the genetic etiology of SCZ might have been overestimated given the inability to detect large genetic effects (
45). Schizophrenia may result from a complex interplay between genetic and environmental factors (
46). For example, HLA genes of the major histocompatibility complex (MHC) have the strongest genetic predisposition for SCZ in genome‐wide association studies (
47). Similarly, MHC genes may affect susceptibility to
Toxoplasma infections (
48,
49). Certain HLA alleles (e.g., HLA C*04:01 allele) have been shown to decrease susceptibility to
T. gondii infection in patients with SCZ but not in controls (
49). Interactions between genetic and environmental risk factors merit further investigation (
46). Environmental factors may be extremely complex and heterogeneous regarding infections by different strains and timing of infection.
Although most studies studied the role of chronic latent toxoplasmosis (IgG seropositivity) with SCZ, approximately a quarter of the studies also studied the potential association of acute toxoplasmosis (IgM seropositivity) with SCZ. On average, 5% of patients with SCZ were
Toxoplasma IgM seropositive, versus 0% of controls. A significant association between
Toxoplasma IgM seropositivty and SCZ was shown in less than 5% of the studies. An earlier meta‐analysis by Monroe et al. (
21) showed that
Toxoplasma IgM seropositivity was associated with an increase in the odds of acute psychosis. However, positive
Toxoplasma IgM results with commercially available tests are often false positive and thus, they cannot be used to diagnose acute
Toxoplasma infection (
50,
51). Positive
Toxoplasma IgM results in commercial labs should always be confirmed with additional tests before the diagnosis of acute toxoplasmosis is made. Studies claiming an association between acute
Toxoplasma infection and SCZ based only on positive
Toxoplasma IgM results, without additional confirmatory testing, should be viewed with caution.
There is speculated biological plausibility for the role of toxoplasmosis in SCZ. Several experimental lines of research addressing parasite‐induced anatomical, histological, and physiological changes have been published; however, there is heterogeneity in reported results (
14). During the chronic latent stage of the infection, formation of bradyzoites in the brain could directly alter the dopamine biosynthesis and cause dopaminergic disturbances involved in psychotic disorders (
52,
53). The elicited anti‐
T. gondii immune responses may cross‐react with host N‐methyl‐D‐aspartate receptors (NMDAR), causing disruption of neural circuits and cognitive deficits (
13). Autoantibodies that bind to the NMDARs may underlie glutamate receptor dysfunction and cognitive impairment found in SCZ (
54). Latent
Toxoplasma infection has been associated with upregulation of the complement C1Q classic immune pathway, which aids in the clearance of the parasite from the central nervous system with subsequent consequences for the connectivity of neighboring cells and synapses, suggested to be involved in SCZ onset (
10). Moreover, complement C4 genes have been proposed in gene‐environment interaction studies as potential susceptibility loci for SCZ and infections (including
T. gondii infections) (
55). Patients with SCZ have increased plasma levels of complement C4 protein activation products, causing increases in blood brain barrier permeability (
56). However, there is heterogeneity in the animal studies about the proposed underlying pathophysiologic mechanisms (e.g., alterations in neurotransmitter release, cyst location, and neuroinflammation) for the association of toxoplasmosis with SCZ (
14,
17,
57,
58). This heterogeneity may arise from differences in the behavioral assays used, the timing of the behavioral assays, the
T. gondii and mice strains utilized, and the route of infection (
14). If
T. gondii influences human behavior or disease, the effect may depend on the genetic background of the individual and the context of the
T. gondii infection (
14).
Several neuroleptic antipsychotic and mood stabilizers have been tested for their ability to inhibit replication of
T. gondii (
59). Among those, the antipsychotic haloperidol and the mood stabilizer valproic acid have been shown to most effectively inhibit
T. gondii growth in vitro (
60). McFarland et al. (
61) and Neville et al. (
62) recently reviewed experimental compounds (
61) and clinical approved drugs (
62) with anti‐
Toxoplasma activity. Chorlton et al. (
63) identified four published RCTs (
64,
65,
66,
67) testing different medications in patients with SCZ. Several important limitations were identified that likely biased the results towards the null; including failure to target specifically
Toxoplasma seropositive patients with SCZ (in 2/4 of these trials) and selecting not clinically appropriate medications. The medications tested in those trials included azithromycin (
64), trimethoprim (TMP) monotherapy (
65), artemisin therapy (
66) and artemether therapy (
67). These medications are not considered first line anti‐
Toxoplasma treatments (
64) and most of those are not even considered acceptable anti‐
Toxoplasma treatments (
65,
66,
67) and have not been used in other clinical settings (e.g., for treatment or prophylaxis of high‐risk
Toxoplasma seropositive patients) (
68,
69). Four additional small RCTs are currently listed in
ClinicalTrials.gov, testing pyrimethamine monotherapy (
70), artemisin plus risperidone (
71), valproate (
72) and L‐tetrahydropalmatine (
73) in patients with SCZ. These trials similarly do not use first line medications and are not targeting only
Toxoplasma seropositive patents with SCZ.
Further association studies are unlikely to offer more solid evidence at this point. We believe that randomized double‐blind placebo‐controlled clinical trials are urgently needed to test the role of anti‐
Toxoplasma primary prophylaxis (with first line anti‐
Toxoplasma prophylaxis medications) in
Toxoplasma seropositive patients with SCZ. These trials should be ideally simple in design, pragmatic, multicenter, and with few clinically important endpoints. The COVID‐19 era has taught us that only well designed, large, RCTs are able to provide solid clinical evidence in a timely fashion and to change our clinical practices (
74,
75).
The hypothesis underlying a study testing a first line anti‐
Toxoplasma prophylaxis medication in
Toxoplasma seropositive patients with SCZ is that prevention of local intermittent subclinical reactivations of
Toxoplasma cysts in the brain of these patients may positively impact their SCZ course. Currently, there are no clinically available medications for the eradication of bradyzoite tissue cysts (the
T. gondii tissue form in chronic latent
Toxoplasma infection) (
76). The goal of such a study in patients with SCZ should be the prevention of intermittent subclinical reactivation rather than eradication of latent
T. gondii infection. This has been the strategy for prophylaxis of immunocompromised patients who are
Toxoplasma seropositive (
69,
77,
78). The proposed mechanism on how anti‐
Toxoplasma prophylaxis may help SCZ is that prevention of subclinical reactivations of
Toxoplasma cysts may prevent secondary alterations in neurotransmitters' release and/or neuroinflammation and subsequent worsening of SCZ clinical course. A proof‐of‐concept for such prophylaxis study will require at least 1 year of prophylactic drug since reactivations may be periodical and spaced over time.
The preferred first line anti‐
Toxoplasma primary prophylaxis regimen is TMP/sulfamethoxazole (TMP/SMX; one double‐strength or one single strength tablet once daily) (
69,
77,
78). Long‐term experience exists for the safety and efficacy of TMP/SMX prophylaxis in several high‐risk immunocompromised patients, for example,
Toxoplasma seropositive transplant patients (
69,
77) or patients with acquired immune deficiency syndrome (
78). The majority of these patients tolerate TMP/SMX without toxicities altering the benefit/risk ratio. Primary prophylaxis in
Toxoplasma seropositive hematopoietic stem cell transplant patients is routinely recommended for at least 6 months or even longer for certain patients considered significantly immunosuppressed‐requiring prophylaxis‐for more prolonged periods (
69,
77). Prolonged secondary prophylaxis with TMP/SMX is also recommended in patients with recurrent toxoplasmic eye disease in vision threatening areas (e.g., for 12–20 months for certain patients) (
79,
80) and for
Pneumocystis jiroveci prophylaxis in severely immunocompromised patients (e.g., up to 6–12 months for certain transplant patients) (
81).
We propose that Toxoplasma IgG seropositive patients with SCZ should be randomized to primary prophylaxis with a first line anti‐Toxoplasma medication such as TMP/SMX versus placebo. The selection of an appropriate first line anti‐Toxoplasma medication, similar to what is routinely used for primary prophylaxis in other clinical settings, is critical. Moreover, the duration of the prophylactic therapy (e.g., at least 1 year) would be an additional key factor to allow for proper assessment of clinically important impact on SCZ course. Multidisciplinary collaboration between psychiatrists, infectious diseases experts, and research methodologists in the design of such a pragmatic clinical trial should be a priority.