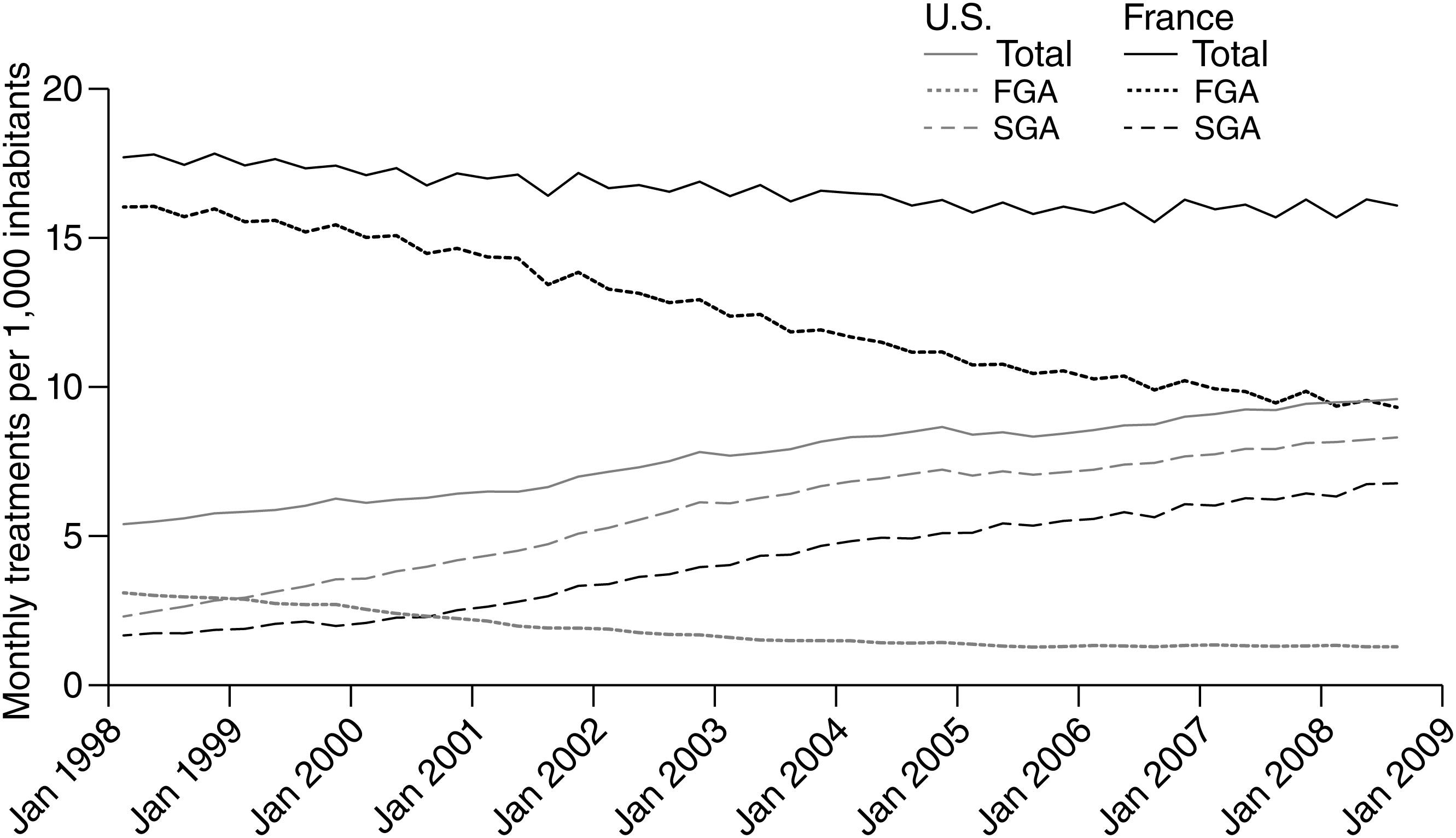
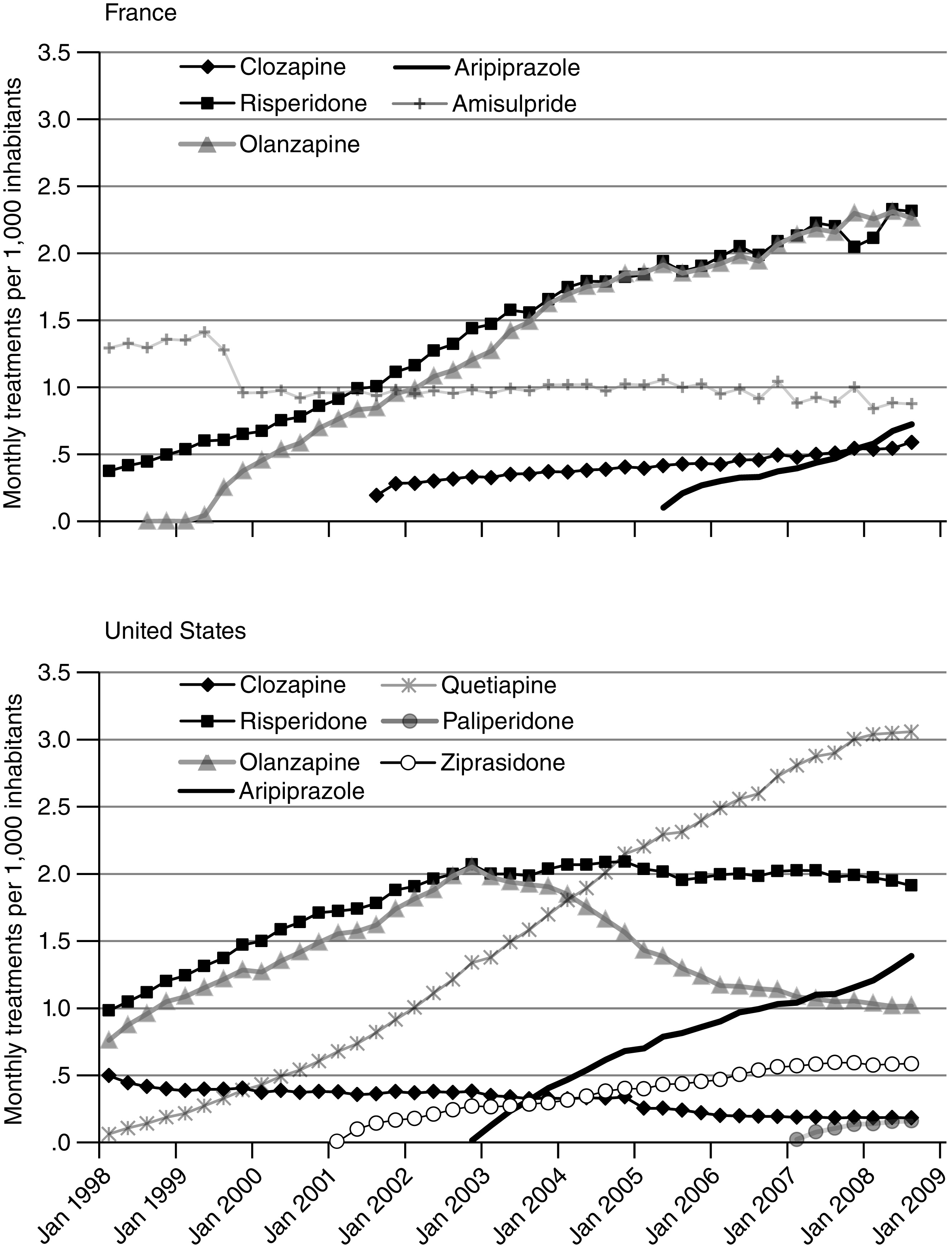
The antipsychotic market in the United States is dominated by second-generation antipsychotics. Seven new drugs introduced between 1989 and 2006 quickly took nearly 90% of the market for antipsychotics and expanded the total number of users of antipsychotic medication, with a substantial portion of expanded use owing to off-label indications (
1–
3). The rapid diffusion of second-generation antipsychotics has been controversial in light of evidence of increased metabolic risks (
4,
5) and their significantly higher costs compared with first-generation antipsychotics (
6–
8).
Other countries have also seen significant increases in use of more costly second-generation antipsychotics (
9–
13); however, few studies have compared trends in the use of antipsychotics in the United States and other countries. We compared trends in use of antipsychotics in the United States with those of France for two reasons. First, according to the World Health Organization, France has the highest-performing health system (
14). Second, physicians in the United States and France had access to a somewhat similar list of approved second-generation antipsychotics but differed with respect to reimbursement policy, use of cost sharing, and other functions.
The study reported here examined trends between 1998 and 2008. We present trends in use overall, by class (first- versus second-generation antipsychotics), and by product. To provide some context for our findings, we briefly present background on the two countries’ health system features that may influence medication utilization. Although a full examination of the impact of these features on antipsychotic diffusion is beyond the scope of this article, we offer some hypotheses in our discussion section as to how antipsychotic use may be shaped by health system and other factors. We also provide information on the drugs available (specifically, drugs approved by regulators) for use in both countries.
Background on the French and U.S. health systems
Health systems support the development and appropriate diffusion of technology through their regulatory approval process, reimbursement policy, postmarketing surveillance of drug safety, and provision of safety information to clinicians and consumers (
Table 1). More detailed overviews of the French system have been published elsewhere (
15,
16). Both pharmaceutical systems have similar drug approval processes and pharmacovigilance systems, and both countries offer coverage for low-income and elderly populations. Conversely, the universality and comprehensiveness of health insurance benefits, the policies regarding drug pricing, and drug promotion regulations differ dramatically between the two countries.
Drug approval and marketing
Approval of new drugs is based on the same criteria in both countries: drug quality, efficacy, and safety. However, whereas drugs are typically marketed by manufacturers shortly after approval by the Food and Drug Administration (FDA) in the United States, commercialization in France does not occur until separate agencies make price-setting and reimbursement decisions.
Coverage, payment, and pricing
The U.S. system lacks universal coverage; approximately 49.9 million individuals were uninsured in 2010 (
17). Insured individuals obtain their coverage through commercial insurers (often employer sponsored) or through public sources such as Medicare or Medicaid. Adults with severe mental disorders are more likely than those without such disorders to be uninsured (21.0% versus 16.5%); if they are insured, they are more likely to receive coverage through Medicare or Medicaid than through commercial insurers (
18). In contrast, France mandates enrollment of all persons in its health system, which includes prescription drug benefits.
Systems for determining reimbursement prices for prescription drugs also differ greatly between the two countries. In the French ambulatory care setting, drug prices are set nationally through negotiation between the health authorities and pharmaceutical firms and depend on the drug’s innovation (
19,
20). Prices paid for pharmaceutical drugs in the United States vary by payer.
In the United States, each payer takes a different approach to coverage of medications, including antipsychotics. For example, Medicare requires the plans with which it contracts to cover “all or substantially all” antipsychotics (
21). Similarly, most Medicaid programs do not restrict coverage of antipsychotic medications, although some states impose prior-authorization requirements on some (
22). Other third-party payers may limit coverage of or impose higher cost sharing for some antipsychotics.
In France, all approved antipsychotics are reimbursed. Under a general rule, patients must pay 35% of the drug cost. However, in practice, few patients pay this share because pharmacy copayments are generally covered by the patient’s supplemental insurance (and 94% had such insurance in 2008) (
23). In addition, patients with chronic psychiatric conditions are offered full coverage for health care costs related to their chronic disease.
In the United States, patients’ out-of-pocket costs vary by payer and drug. Patients enrolled in Medicaid and Medicare beneficiaries who are eligible for low-income subsidies face little to no out-of-pocket cost (
24). For other Medicare beneficiaries, costs vary widely (
22). In 2010, commercially insured patients spent out of pocket an average of $46 monthly for a brand-name second-generation antipsychotic and $12 monthly for a generic form (
25). Uninsured patients are responsible for the full cost of drugs, and large cost differences exist between first-generation antipsychotics and most second-generation antipsychotics (
7).
In France, there is usually no out-of-pocket cost associated with antipsychotic medications apart from a €.50 deductible per package of drugs purchased (up to a limit of €50 per year). Thus patients do not spend more for branded or nonpreferred drugs versus generics, as they typically do in the United States.
Pharmacovigilance
After drugs have been approved, both the FDA and French Agence Nationale de Sécurité du Médicament may issue safety warnings as evidence emerges on a drug’s risk profile. The FDA may require labeling changes to be printed in black box warnings or added to the drug’s monograph. In France the new safety information is added to the drug’s monograph and patient information leaflet found in every drug package.
Information concerning the risks of antipsychotics has been handled differently by the two countries’ regulatory agencies. In September 2003, the FDA issued a warning about the metabolic risks of second-generation antipsychotics. No such warning has been issued in France. Both countries’ regulatory agencies issued warnings about increased mortality risk with second-generation antipsychotics used by older adults with dementia (in March 2004 in France and April 2005 in the United States); these warnings were later expanded in 2008 in both countries to include all antipsychotics.
Promotion
Promotion of pharmaceuticals to health care professionals is regulated in both countries. Promotional materials should be consistent with drug labels and, consistent with FDA and ANSM language, “not be false or misleading”; in particular, promoting off-label use is forbidden. Drug samples and direct-to-consumer promotion of prescription drugs are permitted in the United States but are tightly restricted in France.
Methods
Data sources
For the United States, the data were collected from prescriptions dispensed for oral antipsychotics from IMS Health’s Xponent database. Xponent directly captures over 70% of all U.S. prescriptions filled in retail pharmacies and uses a patented, proprietary projection methodology to represent 100% of prescriptions filled in these outlets. We obtained monthly data on all oral antipsychotic prescriptions that were filled from January 1, 1998, through September 30, 2008, by patients of a 10% random sample of U.S. physicians; these patients filled at least one prescription for an antipsychotic over the period. To extrapolate to all U.S. physicians, we multiplied the prescriptions observed in our data set by 10.
French prescription fills for oral antipsychotics from 1998 to 2008 were extracted from the GERS (Groupement pour l’élaboration et la réalisation de statistiques) database, which collects data on sales to community pharmacies for all pharmaceutical products in France.
Drugs
Antipsychotics were identified by an ATC (anatomical, therapeutic, and chemical) classification code starting with N05A (excluding lithium N05AN). In some analyses, we report product-specific use among the second-generation antipsychotics available in one or both countries by the end of the study period.
Use of injectable antipsychotics was not included because this form is mainly administered in hospitals or physicians’ offices and thus is not recorded in the databases we used.
Analysis
We combined all drug formulations at the ingredient level and report quarterly market shares or rates of use. For both data sources, we converted drug quantities into a monthly unit of treatment (the quantity needed for 30 days of treatment regardless of strength). For the United States, we assumed that a prescription equaled a 30-day supply. Although this assumption might underestimate the total number of prescriptions if a substantial number were filled for a 90-day supply by mail order pharmacies, we did not expect mail order use to vary by class or product. Furthermore, antipsychotics were highly likely to be filled for a 30-day supply because of dispensing limits imposed by most state Medicaid programs (
24), which finance a majority of antipsychotics (
21).
We converted the French data from number of packages sold each quarter into monthly supplies. For second-generation antipsychotics, packages of 30 or 60 tablets of any strength corresponded to a monthly supply (assuming daily intake and that a dispensed package corresponded to a monthly prescription). However, because first-generation antipsychotics’ packages are frequently dispensed for quantities other than a month’s supply (with 20 or 50 tablets), we used 2006 IMS estimates of mean tablets used per day for commonly used first-generation antipsychotics to calculate average monthly supplies (
26).
To determine population-based rates of antipsychotic use, we calculated the number of monthly treatments per 1,000 inhabitants using yearly estimates of population size from the U.S. Census Bureau and its French equivalent (the Institut National de la Statistique et des Études Économiques).
Discussion
Our study offers three main findings. First, the two countries had divergent trends in antipsychotic use overall, with use per population increasing in the United States and declining in France, where the initial level of use was higher. Second, we found large differences between the two countries in the market shares of first and second generations of antipsychotics. By 2008, first-generation antipsychotics accounted for only 14% of antipsychotic use in the United States, while they still made up a majority of use in France. Finally, the product-level market shares for second-generation antipsychotics showed different patterns, with the most notable difference in olanzapine’s share of use in the two countries.
The difference in absolute rates of overall antipsychotic use between the two countries is notable. However, because the data available to us were drawn with different sampling strategies, we were unable to determine whether these rates were truly different, perhaps due to differences in medication access and affordability or whether they were an artifact of the way we measured use. As a result, we focus our discussion on the divergent trends between the two countries.
Physicians in the United States were much more rapid to shift toward use of newer over older antipsychotics than were physicians in France. Because of the huge price differences between first- and second-generation antipsychotics during the study period (differences that will narrow with the launch of generic second-generation antipsychotics), the rapid adoption of newer drugs among U.S. physicians had important economic consequences for payers, particularly Medicare and Medicaid, which finance most antipsychotic prescriptions (
21).
Physicians’ prescribing of antipsychotics was likely influenced by patients’ clinical characteristics, which we were unable to measure. Prescribing was also influenced by physician preferences. The long clinical tradition in France of combining two first-generation antipsychotics (one mainly sedative and one more active on positive symptoms of schizophrenia) (
27,
28) may not have existed in the United States and may explain the higher use of first-generation drugs in France compared with the United States. However, the difference in the speed with which second-generation antipsychotics captured the antipsychotic market in the two countries also may have been influenced by health system factors, some of which we briefly reviewed. For example, the slower adoption of new second-generation antipsychotics in France may be explained by the 2.1-year delay in drug approval. The higher use of second- over first-generation antipsychotics in the United States may also be due in part to the availability of more second-generation antipsychotics and the number of indications for which manufacturers sought regulatory approval. Likewise, the higher use of first-generation antipsychotics in France may reflect the greater number of first-generation drugs available there.
Other findings may not have been predicted on the basis of an assessment of health system differences alone. For instance, there is substantial evidence that choice of medication class is responsive to relative out-of-pocket price (
29). On this factor alone, one might have expected greater use of first-generation antipsychotics in the United States, where there is a steeper gradient in cost sharing between generic and brand-name drugs, compared with France, where most patients face no copay for antipsychotics.
Regulatory agencies and physicians in both countries appear to have responded differently to information regarding the comparative effectiveness and safety of second-generation antipsychotics (
30,
31). The dramatic decrease in olanzapine use in the United States beginning in 2003 coincided with an FDA warning in that year on the metabolic effects of new antipsychotics and with a consensus statement published shortly thereafter ranking olanzapine as having high metabolic risk (
32). In response to these concerns, some state Medicaid programs placed restrictions on second-generation use after the warnings (
22,
33). In contrast, the French drug regulatory agency issued no such warning and did not place any market restrictions on drugs. In addition to differences in the actions of the regulatory agencies, it is possible that French and American physicians differed in their perception of antipsychotic risks. Perceptions of American physicians may have been shaped by the extensive publicity surrounding U.S. lawsuits against olanzapine’s manufacturer (
34).
Our study had some limitations. First, this descriptive study could not generate causal estimates of the relationships between features of the health system and trends in antipsychotic use. Second, the data for the two countries came from different sources: prescriptions (written and filled) from a random sample of physicians in the United States and sales in the ambulatory care market in France. To compare use between the two countries, we used the most comparable unit available from both sources: monthly treatments. However, to convert the number of sold packages in France to the number of monthly treatments, we had to make assumptions about the average daily dose for first-generation antipsychotics because packages are not standardized for monthly treatments. However, although this may have led to some error in estimating differences between countries in first-generation antipsychotic use, we do not expect this measurement error to change over time, and therefore it cannot explain differences in trend. In addition, unlike U.S. data, French sales data capture prescriptions for outpatients and most of the consumption by patients living in nursing homes. Last, we did not record the use of long-acting or other injectable antipsychotics. Hence we were not able to explore differences in the use of the entire class of antipsychotics. The use of long-acting antipsychotics in the early 2000s for patients with schizophrenia has been reported to be similar in the United States (26%) (
35) or France (21%) (
36) and thus should not affect the comparisons.
Acknowledgments and disclosures
Dr. Gallini was supported by a scholarship from the University Hospital of Toulouse, France, and the Direction de la recherche, des études, de l’évaluation et des statistiques, Ministry for Health, France. Dr. Donohue and Dr. Huskamp were partially supported by grant R01MH093359 from the National Institute of Mental Health. Dr. Huskamp was also partially supported by a Robert Wood Johnson Foundation investigator award in health policy research. Dr. Gallini’s access to data on antipsychotic utilization in France from Groupement pour l’Elaboration et la Réalisation de Statistiques was authorized under a contract with Direction de la recherche, des études, de l’évaluation et des statistiques within France’s Ministry for Health. Dr. Donohue and Dr. Huskamp obtained data on U.S. antipsychotic utilization from IMS Health. The authors are grateful to Hocine Azeni, M.A., who provided expert statistical programming. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from IMS Health, Inc., Xponent information services (1996–2008). The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health, Inc., or any of its affiliated or subsidiary entities.
The authors report no competing interests.