More than a decade of research suggests that clozapine is the most effective antipsychotic agent for people with schizophrenia who do not respond adequately to other antipsychotic medications (
1,
2). Despite its benefits, use of clozapine has declined since the introduction of newer antipsychotic medications with fewer serious safety risks, although these new drugs have not demonstrated comparable efficacy for patients with refractory symptoms. Clozapine is often used as a last-resort drug, and it accounts for less than 5% of all antipsychotic prescriptions in the United States (
3,
4). Failure to offer clozapine as a treatment option to patients with refractory symptoms is a major barrier to optimal care.
Clinical guidelines recommend the initiation of clozapine after two inadequate trials of antipsychotic treatment (
5). However, the start of clozapine treatment is often delayed longer than recommended (
6). Studies suggest that the average time between initiating psychiatric treatment and starting clozapine ranges from five to nine years and that some patients receive an average of 3.5 to five or more antipsychotics before clozapine is prescribed (
6,
7). Patterns of clozapine prescribing vary considerably, especially for certain subgroups of patients. Racial and ethnic minority groups, particularly African-Caribbean patients, are less likely than white patients to receive clozapine (
8). Studies of barriers to clozapine use suggest that prescribers are not offering clozapine as a treatment option or delaying its start because they lack experience, are unaware of clozapine’s benefits, perceive blood monitoring as burdensome, and are concerned about potential side effects (
9,
10).
The New York State (NYS) Office of Mental Health (OMH) aims to promote evidence-based prescribing practices, to reduce costly practices that are not supported by evidence, and to ensure that patients taking antipsychotics are monitored effectively for adverse effects. Given the limited access to clozapine and variation in prescribing practices, a better understanding of factors associated with initiating clozapine and with initiating other antipsychotic medications is important for developing initiatives to increase the appropriate and safe use of clozapine.
Methods
We used NYS Medicaid claims data from 2008 to 2009 to identify individuals with a primary diagnosis of a schizophrenia spectrum disorder (ICD-9 codes 295.1–295.3, 295.6, 295.7, and 295.9) who had continuous Medicaid eligibility during the study period and at least one clinic service and an antipsychotic prescription filled in 2009. Individuals who were dually eligible for Medicaid and Medicare were excluded because this group receives prescription benefits through Medicare Part D. We limited the study population to include individuals who initiated a new antipsychotic medication, defined as someone with a filled antipsychotic prescription in 2009 without any fills for the same medication in the 90 days before the start date (N=7,035). Because nearly half of the sample had a filled prescription of the same medication in the year before the 90-day window, we performed a sensitivity analysis for the subgroup without any prescriptions of the same medication in the prior year (N=3,782, 54%). The OMH Institutional Review Board determined that our analysis did not meet the definition of research because it was conducted as part of a quality improvement effort in NYS.
The dependent variable was defined as whether the newly initiated drug was clozapine (coded as 1) or another antipsychotic medication (coded as 0). Predictive factors were demographic characteristics (age, gender, and race), type of facility where the claim was filed (state operated versus other), and clinical variables extracted in the 12 months before the start date of the new drug. The clinical variables included the number of prescriptions filled for different antipsychotic medications, a diagnosis of substance abuse or dependence, the number of inpatient admissions, and total Medicaid psychiatric costs.
For descriptive purposes, we examined the percentage or mean of each independent variable for the groups starting on specific medications. We used t tests and chi square analyses for these comparisons. We used multivariate logistic regression analysis to examine the influence of demographic and clinical factors on initiation of clozapine versus other antipsychotic medications. Statistical analyses were performed with Stata 10.0 statistical software.
Results
The mean±SD age of the total sample (N=7,035) was 44±12 years, and 50.3% (N=3,537) were men. The largest proportion of enrollees were black (N=2,549, 36.2%), followed by Caucasian (N=2,007, 28.5%), Hispanic (N=922, 13.1%), and other racial groups (N=1,557, 22.1%).
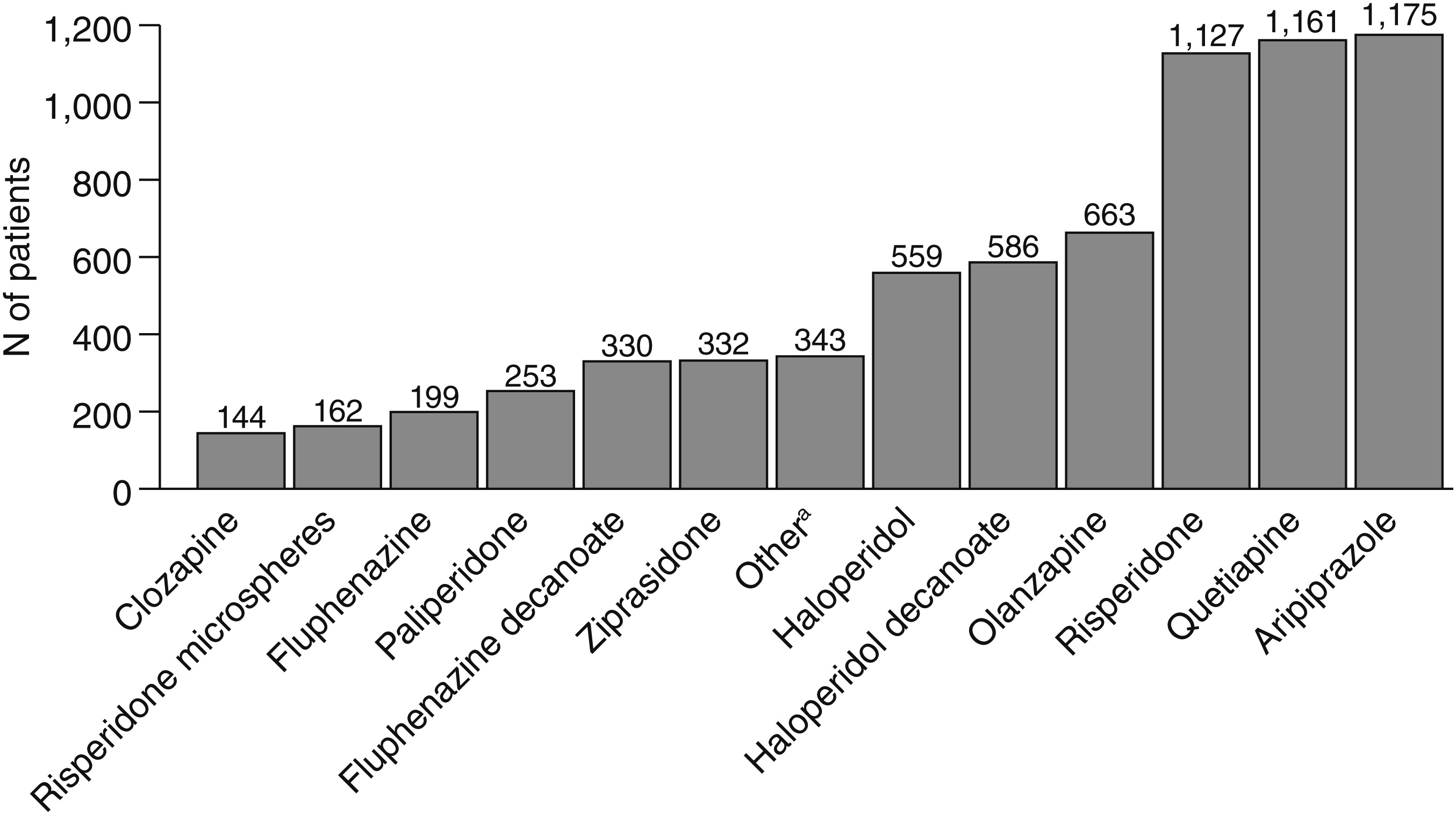
Figure 1 shows the frequency distributions for each new-start antipsychotic medication in 2009. Of the 7,035 patients, 144 (2%) started on clozapine and 6,891 (98%) on another antipsychotic in 2009. Those who started on clozapine were younger than those who started on other drugs (mean±SD ages of 36±12 and 44±13 years; t=7.7, df=7,032, p<.001). Men were more likely to receive clozapine than women—88 men (61.1%) compared with 56 women (38.9%) (χ
2=6.89, df=1, p<.01). Caucasians (N=57, 39.6%) were more likely to start on clozapine than blacks (N=42, 29.2%), Hispanics (N=11, 7.6%), and other racial groups (N=34, 23.6%) (χ
2=11.8, df=3, p<.01).
Of the 448 patients receiving treatment in state-operated facilities, 28 (6.3%) started on clozapine, compared with 116 (1.8%) of the 6,587 patients receiving treatment in other facilities (χ2=11.8, df=3, p<.01). Compared with patients who started on antipsychotic drugs other than clozapine, those who started on clozapine were prescribed a greater number of different antipsychotic medications in the previous year (mean±SD=2.0±1.3 versus 1.5±1.0; t=5.5, df=7,032, p<.001), were more likely to have three or more inpatient admissions in the previous year (36 patients [25%], compared with 613 patients [8.9%]; χ2=74.7, df=2, p<.001), and had higher total Medicaid psychiatric costs in the previous year ($58,861±$65,367 compared with $16,687±$27,767; t=17.3, df=7,032, p<.001). No significant differences between groups were found in diagnoses of substance use disorders.
The multivariate logistic regression analysis showed that starting on clozapine was less likely among older patients than among younger patients (odds ratio [OR]=.96, 95% confidence interval [CI]=.94–.97). Starting on clozapine was also less likely among patients from racial and ethnic minority groups than among Caucasians (black or African American, OR=.57, CI=.38–.86; Hispanic, OR=.47, CI=.24–.91). Having a substance use disorder was associated with significantly lower odds of starting on clozapine (OR=.46, CI=.30–.71). New starts on clozapine were more likely among patients with more hospitalizations (one or two hospitalizations, OR=1.64, CI=1.05–2.59; three or more, OR=1.88, CI=1.03–3.45) and higher psychiatric costs (OR=1.46, CI=1.27–1.69) and among those who received care in state-operated facilities (OR=2.28, CI=1.46–3.56).
The pseudo R
2 for the full model was .13, an approximate assessment of variance in the outcome (
11). In the sensitivity analysis for the subgroup of individuals who had not filled any prescriptions in the past year for the medication on which they started, the direction of the associations between the demographic and clinical risk factors were identical but the findings were not statistically significant (data not shown). Thus the results appear to have been unaffected by our decision to include the broader group of patients.
Discussion
Consistent with findings of previous studies (
3,
4), only a small percentage of individuals with a schizophrenia spectrum disorder initiated clozapine. The 2% rate of new starts on clozapine is very low in light of the estimated 30% of individuals with schizophrenia who experience refractory symptoms.
The multivariate regression results suggest that individuals who started on clozapine were more likely than those who started on other antipsychotics to be younger patients in state-operated facilities, who had more psychiatric hospitalizations and greater total expenditures in the previous year. Clozapine is most often used for patients with chronic schizophrenia who have frequent hospitalizations and high treatment costs (
3,
4). In practice, individuals with schizophrenia who need a medication change are often prescribed numerous other antipsychotics before being offered clozapine. Given the personal and societal costs associated with poorly controlled schizophrenia, an important question is whether clozapine should be introduced earlier in treatment (
9,
12).
In light of existing evidence that suggests beneficial effects of clozapine for patients with schizophrenia and a co-occurring substance use disorder (
13), it seems counterintuitive that substance use disorders were negatively associated with initiation on clozapine. One explanation is that clinicians may be hesitant to prescribe clozapine to persons who abuse substances because they do not consider them reliable enough to follow through with regular blood draws. In addition, prescribers may not be aware of the potential benefits of clozapine for patients with a comorbid substance use disorder.
This study found disparities in clozapine use among racial and ethnic minority groups. Copeland and colleagues (
8) found reduced access to clozapine among blacks and Hispanics with schizophrenia. One explanation for some of the disparity is that members of certain racial-ethnic groups may be more likely than whites to have neutrophil counts below the threshold required to start clozapine. This phenomenon, which is common among individuals of African and Middle Eastern descent and has been called “benign ethnic neutropenia,” is an artifact of using white populations to define normative ranges and is not associated with increased rates of or impaired response to infection (
14). Cultural factors, provider concerns about increased risk of diabetes and other side effects in racial-ethnic minority populations, and provider biases are among other possible explanations for racial-ethnic disparities in clozapine use. Although our model accounted for only a small portion of the variance in initiation on clozapine, these findings point to the need for further research on the use of clozapine among racial and ethnic minority groups and people with substance use disorders.
Conclusions
The findings underline the continued dramatic underuse of clozapine to treat individuals with schizophrenia. Clozapine accounted for only 2% of antipsychotic medications initiated in 2009 and paid for by Medicaid in New York State. Multiple factors inhibit the use of clozapine, including clinician reluctance; fear of serious adverse effects, including agranulocytosis; and the perceived burden of monitoring and administration (
5,
6). The perception that patients will not accept the required blood-drawing schedule is widespread (
4,
10).
A better understanding of these factors and other clinician, patient, and system factors associated with clozapine’s underuse is needed to help treatment programs optimize clozapine treatment. Investigation into the causes of disparities in clozapine use also is needed. Quality improvement efforts, such as those designed to give information and provide support to patients (
15) and those designed to train clinicians in the effective use of clozapine, are needed to ensure that appropriate patients are being offered this effective treatment.
Acknowledgments and disclosures
The authors gratefully acknowledge the input and work of Sheila A. Donahue, M.A., and Carol Barth Lanzara, M.S., J.D., in the development of this work.
The authors report no competing interests.