Attention-deficit hyperactivity disorder (ADHD) affects 5% of school-age children worldwide (
1). In the United Kingdom, the prevalence of ADHD as assessed by diagnostic criteria in the general population is estimated at 3.6% for boys and .9% for girls (
2), and the annual prevalence of pharmacologically treated ADHD among children ages six to 12 is .92% (
3). ADHD, characterized by core symptoms of hyperactivity, impulsivity, and inattentiveness, is associated with academic difficulties, poor social relationships, substance abuse, and psychiatric comorbidities in childhood (
4,
5) and across the life span (
6).
Pharmacotherapy (primarily stimulant medication) is effective for the short-term reduction of ADHD symptoms (
7) and for the long-term reduction of poor outcomes associated with ADHD (
8). ADHD treatment guidelines suggest a combination of behavior therapy, parental training, and medication use for as long as clinically effective (
9). Medication adherence, defined as the extent to which a person’s medication-taking behavior (timing, dosing, and frequency) corresponds with recommendations from a health care provider (
10), has been characterized as limited among U.S. children with ADHD (
11). Specifically, medication persistence, defined as the duration of time from initiation to discontinuation of drug therapy (
10), has been estimated as less than six months for stimulants (
12–
16). Given the chronic nature of ADHD, long-term ADHD treatment effectiveness may be complicated by poor medication adherence (
17).
ADHD medication adherence is associated with child factors (such as age, gender, and disease severity) (
18,
19), parental factors (such as parental beliefs about pharmacotherapy) (
17,
20,
21), and treatment factors (such as type, dosage, and side effects) (
12–
16). In particular, once-daily long-acting formulations have been consistently associated with longer treatment duration and continuity compared with the standard formulation (
14–
16,
22).
Although ADHD diagnosis in the United Kingdom tends to be reserved for more severely affected children (
23), pharmacological treatment of ADHD is initiated more cautiously than in North American populations (
24). In the United Kingdom, the incidence of pharmacologically treated ADHD among children younger than 14 (
3,
25) and ADHD treatment cessation among those ages 15 to 21 (
26) have been described. However, children of all ages (≤18 years old) who have been diagnosed as having ADHD have not been described in the United Kingdom nor have treatment patterns for prescribed medication for this group. Therefore, the aims of this study were to describe initial pharmacological treatment patterns among U.K. children with ADHD and to evaluate independent predictors of persistence with initial ADHD treatment.
Methods
Study Population
This study was conducted with data (January 1, 1993, to June 30, 2008) from The Health Improvement Network (THIN) in the United Kingdom. General practices that are part of THIN maintain electronic patient records. Because the general practitioner functions as a gatekeeper to the U.K. national health care system, THIN captures longitudinal patient data about general practice visits, prescriptions, and referrals to secondary care. Information about specialists and hospitalizations (treatment outcomes and discharge reports) are included on the basis of correspondence from other facilities and professionals. The 2008 THIN data set describes 2.3 million patients actively registered with 386 general practices. The THIN data set is obtained from 4% of the U.K. population, and the age, gender, and geographic distributions are similar to those in the overall U.K. population (
27).
The study population included all children ages three to 16 with an incident diagnosis of ADHD between January 1, 1994, and June 30, 2006. To ensure that the ADHD diagnosis did not reflect a diagnosis recorded upon registration in the database, only individuals registered at least one year before the initial ADHD diagnosis were included. Children were excluded if they received an ADHD medication prescription in the year before diagnosis, had less than two years of follow-up after diagnosis, or had less than one year of follow-up after initial medication prescription. Patients were followed from one year after registration to the patient’s 19th birthday, the last data collection date, death date, or date of transfer out of the practice, whichever was earliest.
THIN uses the Read clinical classification system. The definition of ADHD in this study included any Read code that refers specifically to ADHD, attention-deficit disorder, or hyperkinetic disorder, in addition to two very common ADHD-related codes (“reduced concentration” and “short attention span”). These two codes were included to allow for a more accurate assessment of the timing of initial diagnosis, because preliminary analysis indicated that about 90% of patients with these less definitive codes went on to receive a more formal diagnosis within six months. [A list of Read codes used to define ADHD is included in an online supplement to this article.]
ADHD Medication and Treatment Course
All medications approved for ADHD treatment in the United Kingdom during the study period were included (methylphenidate [MPH], dexamphetamine, and atomoxetine). Children were considered pharmacologically treated if one or more prescriptions for ADHD medication were recorded within the first two years after the initial diagnosis.
Two measures of persistence with ADHD medication were described: initial treatment course duration; and for children with more than one treatment course, the length of gaps between treatment courses. Initial treatment course duration was defined as the time from the initial medication prescription date to the end of the treatment period of the last ADHD medication prescription. For the primary analysis, a 30-day grace period was used to determine treatment periods, as in previous studies of ADHD treatment persistence that used administrative data (
13–
15,
22). For 82% of the prescriptions that had values for daily dose and prescription quantity, the number of days supplied by the prescription was between 28 and 32 days. Therefore, missing “days supplied” values were set to 30 days, which also is the recommended prescription length for controlled drugs (such as stimulant medication) in the United Kingdom (
28).
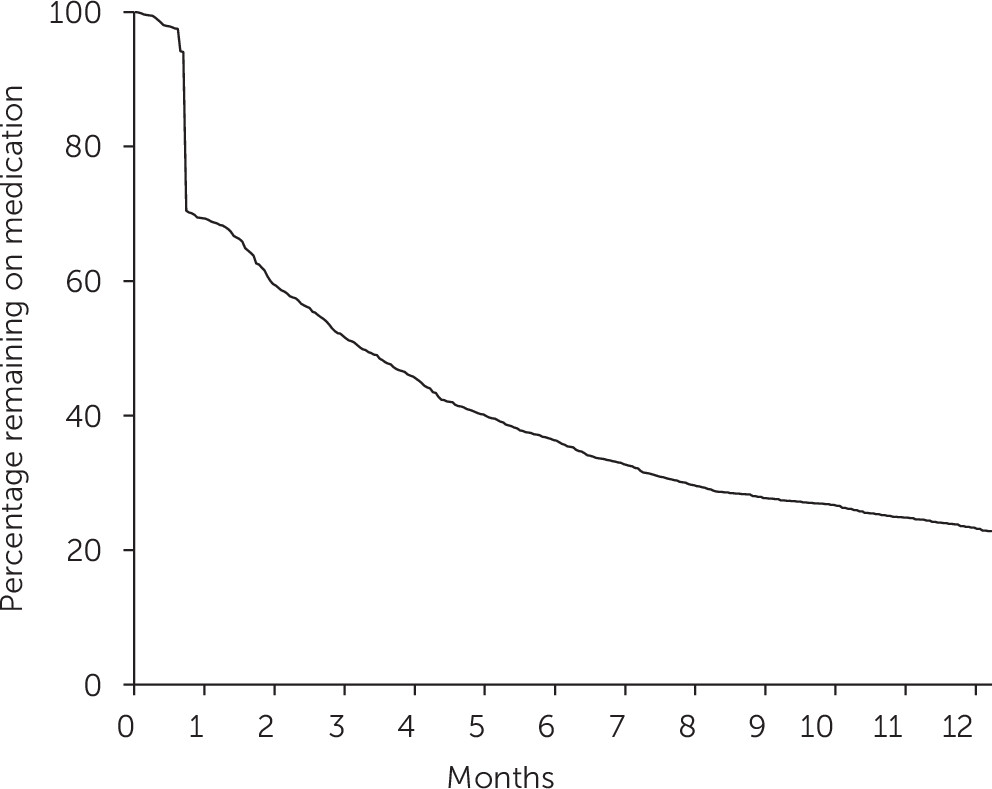
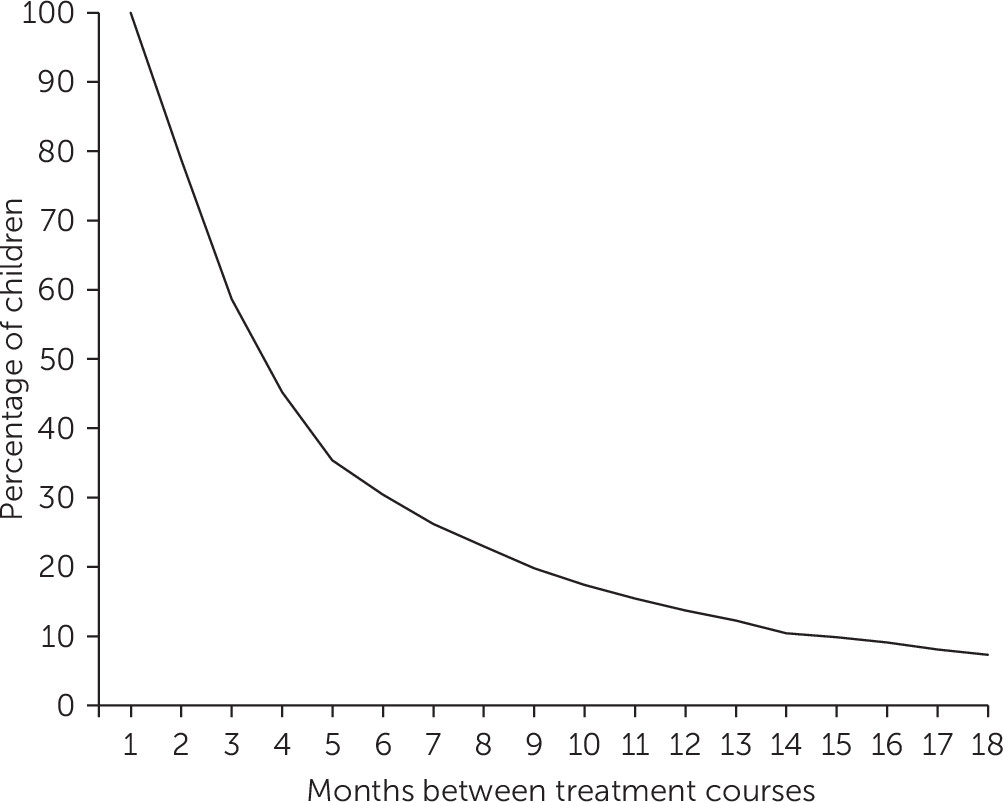
On the basis of the initial treatment course duration, children were classified as persistent (initial treatment course of more than six months) or nonpersistent (initial treatment course of six or fewer months). This six-month cut point was chosen to allow sufficient time for medication use after an effective dose was established. For children with more than one treatment course, the proportion of children with gaps of various lengths between treatment courses was calculated.
Category Definitions
Year of diagnosis was categorized as either before or after 2002, because prescribing practice (and medication persistence) may have changed after the October 2000 publication of the first U.K. guideline on the use of ADHD drugs (
23). Socioeconomic status was categorized by using the Townsend index, which indicates deprivation by use of quintiles from least to most deprived based on the each child’s home postal code (
29). Preexisting diagnosed psychiatric comorbidity was defined as the presence of at least one Read code for anxiety, depression, or conduct disorder (including oppositional defiance disorder) before the diagnosis date. To assess psychiatric comorbidity as a predictor of treatment discontinuation, it was necessary that the diagnosis of comorbidity occur temporally prior to the diagnosis date.
Evidence of specialist involvement was determined by the source of the initial ADHD diagnosis records (for example, specialist or hospital consultant) or the presence of records for referrals made or consultant letters received on the initial diagnosis date.
Analysis
Proportions and cross-tabulations were used to describe children with ADHD by gender, age at diagnosis, year of diagnosis, socioeconomic status quintile, preexisting psychiatric comorbidity, and specialist contact on the diagnosis date, stratified by whether children were treated pharmacologically. Chi square tests were used to explore differences. Time from diagnosis to pharmacological treatment, initial medication type, and time between treatment courses were also calculated for children who received pharmacotherapy. Kaplan-Meier survival analysis was used to describe the initial treatment course duration.
Persistence with medication treatment was calculated by using a 30-day grace period, as well as 60- and 90-day grace periods. Binomial regression was used to evaluate independent predictors of persistence with medication treatment (initial treatment course greater than six months or six months or less). Risk ratios (RRs) and 95% confidence intervals (CIs) were estimated for the association between persistence and child covariates (gender, age at diagnosis, year of diagnosis, socioeconomic status, and psychiatric comorbidities) and clinical covariates (specialist contact on the diagnosis date, first medication type, and time from diagnosis to medication initiation). Analyses were conducted with SAS, version 9.2.
The University of North Carolina Public Health and Nursing Institutional Ethics Review Board approved this study.
Discussion
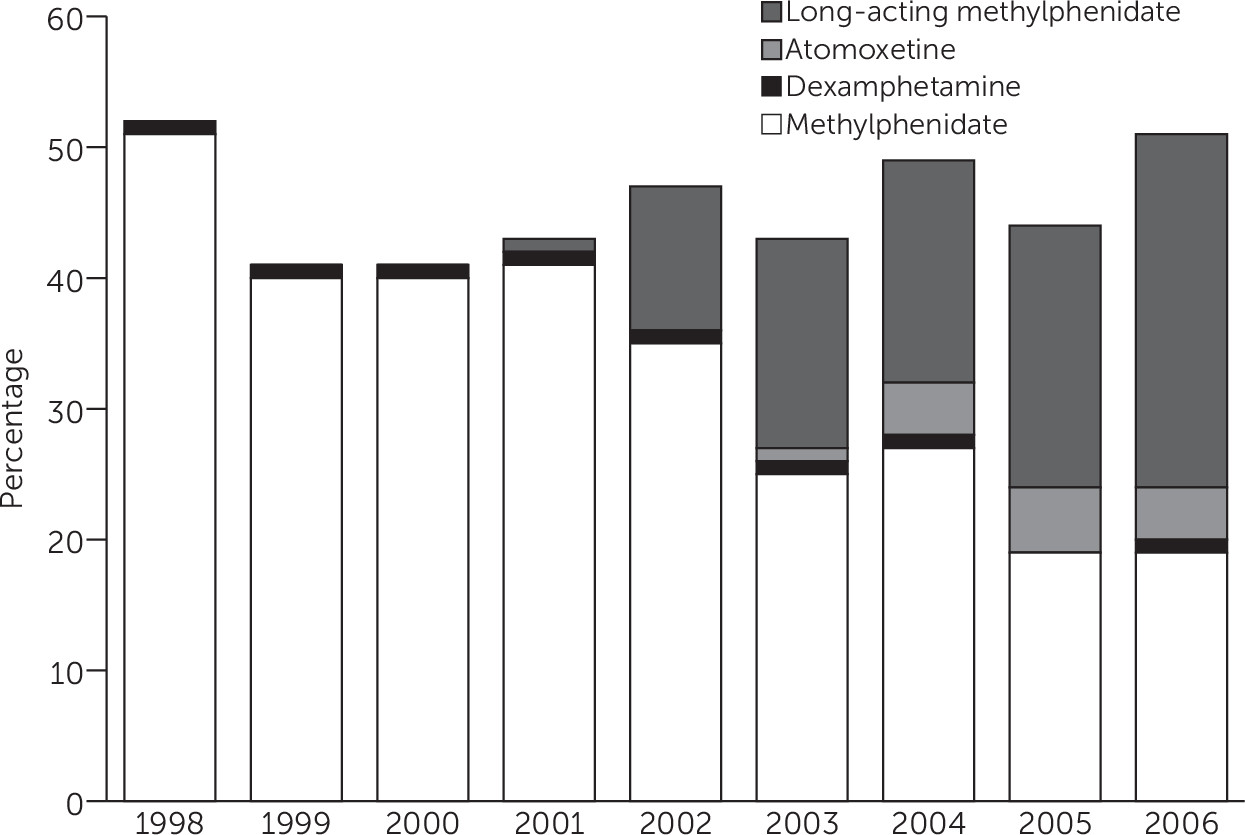
These results provide three novel insights about the pharmacological treatment of U.K. children diagnosed as having ADHD. First, the profile of the study group, in particular the age at diagnosis and the type of medication utilized, changed considerably between 1995 and 2008. Second, only a minority of children (35%) prescribed ADHD medication were persistent with treatment six months after initial prescription when the analysis used a 30-day grace period, and just over half (57%) were persistent when a less stringent 60-day grace period was used. Finally, initiating treatment with long-acting MPH rather than the standard formulation was associated with persistence at six months, when the analysis controlled for a range of child and clinical factors.
Trends shown in the study cohort correspond to changes in how ADHD-related disorders were diagnosed and treated over the study period in the United Kingdom. The increase in the mean age at diagnosis may be related to the decline of the
ICD-based diagnosis of “hyperkinetic disorder” and an increasing acceptance of the
DSM-IV ADHD diagnosis and treatment (
23,
24). The
DSM-IV criteria are less stringent in the number and severity of symptoms required for ADHD diagnosis. Children with less severe symptoms may be older when symptoms are recognized (
30); therefore, inclusion of these children in later years may have increased the mean age at diagnosis. The observed shift in the type of medication prescribed likely reflects changes in the medications available over the study period. Following the relicensing of MPH for ADHD treatment in 1993 (
23), long-acting MPH and atomoxetine became available for use in ADHD in 2002 and 2004 (
9).
To our knowledge, this is the first study to examine ADHD treatment persistence among U.K. children of all ages. In North America, persistence with stimulant treatment has been estimated extensively, with a range of operational definitions for persistence (
12–
16,
31). Using a 30-day grace period, one study found that 50% of new users of MPH continued to use medication at three months (
13), and another found that 47.4% persisted at six months (
31). When the grace period was defined as 20% of the previous prescription length, 84% of children had discontinued treatment by two months (
12). These estimates vary somewhat from the results reported here; however, all indicate that a substantial proportion of children who initiate pharmacological treatment do not continue beyond six months. In the United Kingdom, when a six-month gap was used to indicate treatment discontinuation, the median treatment time among individuals ages 15 to 21 was 1.8 years (CI=1.04–2.56) (
26). The results of the study reported here complement the existing research to show that persistence with ADHD medication may be problematic across age groups in the United Kingdom.
The results regarding gaps between treatment courses suggest that a substantial proportion of children restart treatment soon after the end of the first treatment course. However, many other children restart medication after long periods without treatment, confirming the findings of others (
13,
32). Discontinuation and reinitiation of ADHD medication may be attributed to clinical reasons (for example, as a result of adverse effects, for assessment purposes, or the need for different levels of symptom control at school, at home, and during holidays) or reasons that are less clinically oriented (for example, medication cost or dosing or parental beliefs about medication) (
33). It is estimated that 50%−60% of children diagnosed as having ADHD remain symptomatic during adolescence (
30). Therefore, symptom resolution in the short term is not a likely explanation for most treatment discontinuations. Further research is warranted to understand the relative contribution of these factors to the observed patterns of medication persistence.
The independent association between medication type and persistence with initial treatment beyond six months is consistent with recent literature (
13,
15,
16,
22). Children who are prescribed once-daily, long-acting formulations of MPH tend to be more persistent with treatment, perhaps because this formulation eliminates the need for multiple doses and taking medication at school (
33). In addition, if long-acting formulations have a better effect on symptoms throughout the day, persistence may be enhanced (
34). Unmeasured differences in how and to whom the various formulations are prescribed may also confound this association. For example, clinicians may prescribe long-acting MPH to children with more severe symptoms, who may also have more motivation to continue treatment. However, regardless of medication type, most children were not persistent with treatment at six months when the analysis used a 30-day grace period. Few other factors were associated with persistence in this study, perhaps indicating that factors not easily measured in administrative databases, such as patient and family attitudes regarding medication and concurrent nonpharmaceutical treatment, may provide more insight as predictors of persistence (
35).
The results of this study should be considered in the context of the following limitations. First, the measurement of ADHD may have varied because the diagnostic criteria used by each general practitioner to assign ADHD codes were unknown. However, it is likely that children in this study were diagnosed formally because most had specialist contact around the time of initial diagnosis. The exclusion of children not diagnosed as having ADHD prior to medication prescription allowed better definition of the cohort, but it may have limited the generalizability of the results. Similarly, exclusion of children on the basis of available follow-up time may have biased results if there were consistent reasons why medication was discontinued. For most children who were excluded on the basis of follow-up time, the reason was either being diagnosed in the most recent two years of the database or turning 19 years of age less than two years after diagnosis. These children would be less likely than those who were excluded because of death or transferring out to differ systematically from the study group. The grace period used (30 days) may have affected persistence results. Varying the grace period to 60 or 90 days changed the proportion of children who were persistent in treatment at six months; however, the predictors of persistence did not change substantially. In addition, because prescription records are not directly indicative of whether prescriptions are filled or consumed, the study measures are proxies for actual medication treatment persistence.
This study reflects only primary care for ADHD. U.K. guidelines have recommended that specialists (pediatricians and psychiatrists) diagnose ADHD and initiate medication, communicating with general practitioners to prescribe ongoing medication under “shared care” arrangements (
23). However, guideline adherence is varied and may be related to the local availability of child mental health specialty services (
36). This study could not assess medications that were prescribed from secondary or tertiary care providers and could not describe children who exclusively received medication from specialists. There is some indication that most children who are prescribed ADHD medication by general practitioners are monitored by specialist services as per U.K. guidelines (
36). For those who are prescribed ADHD medications in primary care, the general practitioner would likely provide a majority of prescriptions; however, the division of ADHD medication prescription between specialists and general practitioners is not known. This limitation underscores the importance of further research to detail the reasons for the short and intermittent periods of medication treatment observed in U.K. primary care. The frequency of comorbid conditions among children with ADHD is likely underestimated in this primary care data set. Finally, the most recent study data are from 2008, after which shared care protocols have increasingly been implemented. More recent data would likely produce slightly different results.