Geographic and Clinical Variation in Clozapine Use in the United States
Abstract
Objective
Methods
Results
Conclusions
Methods
Study population
Predictor variables
Statistical analysis
Results
| Clozapine (N=15,524) | Other antipsychotic (N=614,285) | ||||
|---|---|---|---|---|---|
| Characteristic | N | % | N | % | p |
| Sex | <.001 | ||||
| Male | 8,941 | 57.6 | 309,014 | 50.3 | |
| Female | 6,583 | 42.4 | 305,262 | 49.7 | |
| Age | <.001 | ||||
| 18–24 | 1,566 | 10.1 | 44,607 | 7.3 | |
| 25–34 | 3,414 | 22.0 | 110,083 | 17.9 | |
| 35–44 | 4,846 | 31.2 | 190,027 | 30.9 | |
| 45–54 | 4,072 | 26.2 | 182,162 | 29.7 | |
| 55–64 | 1,626 | 10.5 | 87,406 | 14.2 | |
| Race-ethnicity | <.001 | ||||
| White, non-Hispanic | 10,025 | 64.6 | 325,328 | 53.0 | |
| African American, non-Hispanic | 2,871 | 18.5 | 168,201 | 27.4 | |
| Hispanic | 805 | 5.2 | 42,977 | 7.0 | |
| Other | 10,025 | 64.6 | 325,328 | 53.0 | |
| Past-year co-occurring disorder or condition | |||||
| Substance use disorder | 2,003 | 12.9 | 90,848 | 14.8 | .03 |
| Depression | 5,583 | 36.0 | 223,975 | 36.5 | .44 |
| Anxiety | 2,426 | 15.6 | 96,938 | 15.8 | .74 |
| Deliberate self-harm | 202 | 1.3 | 6,560 | 1.1 | .06 |
| Diabetes or cardiovascular disease | 7,742 | 49.9 | 311,178 | 50.7 | .60 |
| HIV | 86 | .6 | 11,487 | 1.9 | <.001 |
| Schizophrenia subtype | <.001 | ||||
| Schizophreniform | 748 | 4.8 | 21,204 | 3.5 | |
| Schizoaffective | 6,792 | 43.8 | 256,349 | 41.7 | |
| Past-year acute services | |||||
| Mental health emergency service | 2,003 | 12.9 | 90,848 | 14.8 | <.001 |
| Outpatient visits for schizophrenia | <.001 | ||||
| 0–9 | 3,970 | 25.6 | 236,786 | 38.6 | |
| 10–29 | 4,122 | 26.6 | 176,163 | 28.7 | |
| 30–49 | 2,254 | 14.5 | 66,137 | 10.8 | |
| ≥50 | 5,178 | 33.4 | 135,199 | 22.0 | |
| Hospital admissions for psychiatric illness | <.001 | ||||
| 0 | 6,902 | 44.5 | 353,514 | 57.6 | |
| 1 | 3,781 | 24.4 | 134,811 | 22.0 | |
| 2 | 2,049 | 13.2 | 59,275 | 9.7 | |
| 3 | 1,069 | 6.9 | 26,982 | 4.4 | |
| ≥4 | 1,723 | 11.0 | 39,703 | 6.5 | |
| Treatment resistance | <.001 | ||||
| Present | 4,367 | 28.1 | 75,567 | 12.3 | |
| Absent | 11,157 | 71.9 | 538,718 | 87.7 | |
Geographic variation
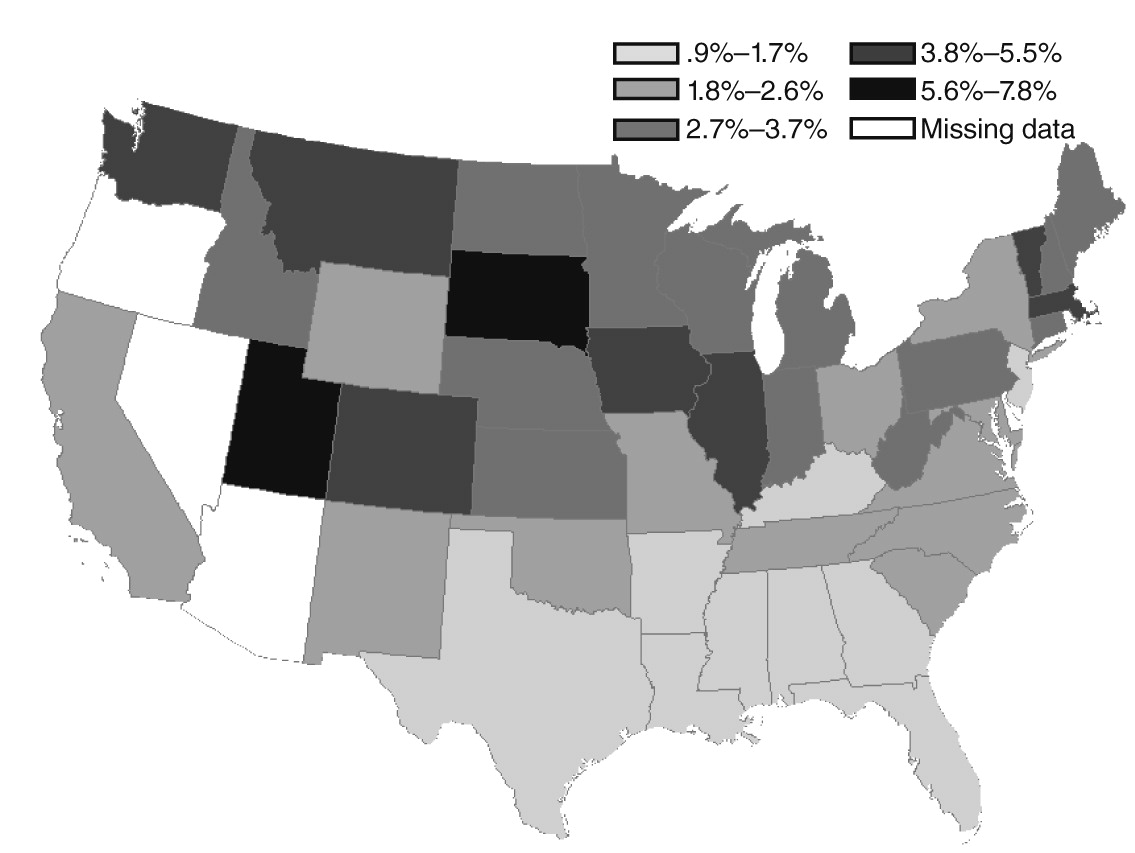
Demographic and clinical predictors of clozapine initiations
| Patient group | AOR | 95% CI |
|---|---|---|
| Male (reference: female) | 1.26 | 1.22–1.30 |
| Age (reference: 55–64) | ||
| 18–24 | 1.81 | 1.62–2.02 |
| 25–34 | 1.53 | 1.40–1.67 |
| 35–44 | 1.29 | 1.19–1.41 |
| 45–54 | 1.15 | 1.09–1.22 |
| Race-ethnicity (reference: white, non-Hispanic) | ||
| African American, non-Hispanic | .663 | .61–.72 |
| Hispanic | .788 | .71–.87 |
| Other | .889 | .84–.94 |
| Diagnosis (reference: schizophrenia) | ||
| Schizophreniform | .93 | .83–1.05 |
| Schizoaffective | .91 | .86–.97 |
| Substance use disorder diagnosis, past year (reference: absent) | .71 | .65–.76 |
| Deliberate self-harm, past year (reference: absent) | .98 | .85–1.13 |
| Diabetes diagnosis, past year (reference: absent) | .90 | .86–.95 |
| Cardiovascular diagnosis, past year (reference: absent) | 1.03 | 1.00–1.07 |
| HIV diagnosis, past year (reference: absent) | .42 | .35–.50 |
| Mood stabilizers, past year (reference: absent) | 1.55 | 1.47–1.62 |
| Long-acting injectable antipsychotic, past year (reference: absent) | 1.16 | 1.06–1.27 |
| Mental health emergency service use, past year (reference: none) | .96 | .90–1.04 |
| Outpatient visits for schizophrenia, past year (reference: 0–9 visits) | ||
| 10–29 | 1.32 | 1.23–1.42 |
| 30–49 | 1.77 | 1.57–1.98 |
| ≥50 | 2.06 | 1.82–2.33 |
| Mental health hospital admissions, past year (reference: 0) | ||
| 1 | 1.19 | 1.11–1.27 |
| 2 | 1.36 | 1.25–1.48 |
| 3 | 1.51 | 1.35–1.68 |
| ≥4 | 1.62 | 1.41–1.87 |
| Treatment resistance (reference: absent) | 1.92 | 1.83–2.03 |
Predictors of clozapine use by patient county characteristics
| Patient group | AOR | 95% CI |
|---|---|---|
| Clozapine-treated patients in county, % (reference: 0%–5%) | ||
| Low (5%–10%) | 1.26 | 1.12–1.42 |
| Medium (10%–15%) | 1.71 | 1.52–1.94 |
| High (>15%) | 2.03 | 1.75–2.30 |
| Psychiatrists per 100,000 residents (reference: 0) | ||
| Medium (.01–14.90) | .97 | .88–1.07 |
| High (≥15) | 1.17 | 1.03–1.33 |
| Annual income per capita, county (reference <$25,000) | ||
| Medium ($25,000–49,999) | .99 | .87–1.12 |
| High (≥$50,000) | .84 | .69–1.02 |
| County population in poverty, % (reference: 0%–14.9%) | ||
| Medium (15.0%–19.9%) | .96 | .87–1.06 |
| High (≥20%) | 1.01 | .86–1.19 |
| County population per square mile (reference: ≤399) | ||
| Medium (400–1,000) | 1.08 | .98–1.20 |
| High (>1,000) | 1.005 | .90–1.12 |
Predictors of clozapine use among treatment-resistant patients
Discussion
Conclusions
Acknowledgments and disclosures
Supplementary Material
- View/Download
- 116.77 KB
References
Information & Authors
Information
Published In

Cover: Snowbound, by N. C. Wyeth, 1928. © Copyright 2014 National Museum of American Illustration™, Newport, Rhode Island. Photo courtesy Archives of the American Illustrators Gallery™, New York.
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
