In June 2003, the U.S. Food and Drug Administration (FDA) released a public health advisory recommending that children treated for major depressive disorder not be given paroxetine because it might increase suicidal thoughts and behaviors (
1). In October 2004, the FDA added a black-box warning to all antidepressants (
2) on the basis of an FDA-sponsored meta-analysis that found that children randomly assigned to antidepressants had twice the rate of suicidal ideation and behavior compared with children randomly assigned to placebo (
3,
4). On the basis of additional analyses, the black-box warning was expanded in May 2007 to include patients from 18 to 24 years old (
5). The warning included recommendations for close patient monitoring after antidepressant initiation (
2).
Following the black-box warning, there were changes in the use of antidepressants among youths—antidepressant use overall decreased (
6–
16), paroxetine use decreased (
7,
11,
14), and fluoxetine use increased (
11,
15). Care of children shifted from generalists to psychiatric specialists (
6,
8,
17), with increased patient referrals to specialists, and the number of new pediatric depression diagnoses decreased (
9,
10). The black-box warning contained recommendations for improved patient monitoring after antidepressant initiation, but it did not lead to significant changes in visit frequency after the warning (
11,
18), although physicians reported increased contact with patients who initiated antidepressants as a change in practice following the black-box warning (
8,
17).
Practice parameters issued by the American Academy of Child and Adolescent Psychiatry (AACAP) in 2007 stated that children and adolescents with depressive disorders should initiate antidepressant treatment with a low dose (
19). As early as 2005, prescribing guidelines from other countries suggested initiating selective serotonin reuptake inhibitors (SSRIs) among children (
20,
21), as well as adults (
22,
23), with low doses, followed by gradual dose titration, given evidence suggesting that antidepressant side effects may be dose related (
24–
26). A higher initial antidepressant dose may be associated with an increased risk of treated self-harm among children and young adults (ages 10 to 24) (
27,
28), highlighting the potential importance of initiating therapy with lower doses, especially among children and young adults. Despite these recommendations, it was uncertain whether starting with a low dose and slowly titrating upward became a more frequent antidepressant treatment strategy and, if so, whether changes in prescribing practices were more pronounced among children than among adults.
To our knowledge, the only published assessment of whether the black-box warnings affected prescriber management of antidepressants through dose alterations was a survey of practicing pediatricians in Canada, 11% of whom reported that the black-box warning affected their management of depression through medication dosing (
29). This study examined directly whether prescribers in the United States may have begun to exert extra caution in terms of initial dosing and subsequent dose titration following the FDA actions, especially among children and young adults. We did so by assessing whether the initial SSRI dose per day prescribed decreased and whether the proportion of children and young adults augmenting SSRI dose on the second fill increased after the 2004 black-box warning.
Methods
Data Source and Study Population
We used the LifeLink Health Plan Claims Database, purchased from IMS Health, which contains data from over 98 health plans throughout the United States. The database includes ICD-9-CM inpatient and outpatient diagnoses; Current Procedural Terminology, Fourth Edition (CPT-4) and Healthcare Common Procedure Coding System (HCPCS) procedure codes; and records for reimbursed, dispensed retail and mail order prescriptions. Records of dispensed prescriptions include the National Drug Code (NDC), quantity, days’ supply, and date of dispensing. The study was deemed exempt by the University of North Carolina Institutional Review Board.
The study population consisted of commercially insured individuals initiating SSRI treatment between January 1, 2000, and December 31, 2009. Children (ages 5–17), young adults (18–24), and, for comparison, adults (25–64) were included. SSRIs were limited to citalopram, fluoxetine, paroxetine, and sertraline; escitalopram was excluded, given that it was not approved by the FDA until late 2002, and therefore there was not sufficient information about dosing before the initial paroxetine advisory. Eligibility criteria also included at least one year of insurance coverage prior to SSRI initiation, no record of antidepressant use in the prior year, six months of insurance coverage following the initial prescription to help ensure that a second prescription would be captured, and a valid initial dose per day.
Dose Per Day and Dose Augmentation
For each SSRI prescription, dose per day was calculated on the basis of days’ supply, strength (from NDC code), and quantity dispensed (11,704 [2.2%] patients in the study population were excluded because of missing values). A valid initial dose per day had an amount per day value (defined as quantity divided by days’ supply) from .5 to 4.0 pills per day for the tablet form and .25 ml to 20 ml per day for the solution form and a dose-per-day value between 2.5 mg/day and 1.5 times the recommended maximum therapeutic dose per day (citalopram, 60mg; fluoxetine, 120 mg; paroxetine controlled release [CR], 93.75mg; paroxetine immediate release [IR], 90mg; and sertraline, 300mg). We excluded 3,151 patients (.6%) with invalid initial dose-per-day values.
We defined low dose by age group and SSRI agent on the basis of available guidelines and product labels (
22,
23,
30–
38), with low dose defined as less than the recommended initial dose: ages 5–12 (<10 mg/day of citalopram, fluoxetine, and paroxetine IR and <25 mg/day of sertraline), ages 13–17 (<20 mg/day of citalopram and fluoxetine, <10 mg/day of paroxetine IR, and <50 mg/day of sertraline), and ages 18–64 (<20 mg/day of citalopram, fluoxetine, and paroxetine IR; <25 mg/day of paroxetine CR; and<50 mg/day of sertraline). A low dose for paroxetine CR for children (5–17 years) was defined as <12.5 mg/day, but this dose per day is unavailable. Because paroxetine was the only agent in which the low dose differed for children ages 13–17 and young adults, we conducted a sensitivity analysis by using an alternative definition for low-dose paroxetine (IR, <20 mg/day; CR, <25 mg/day) for children ages 13–17.
Dose augmentation was defined as an increase of >1 mg between the initial dose per day and the dose per day of the second prescription. To be eligible for a dose augmentation, patients were required to fill a second prescription for the same agent within the initial prescription’s days’ supply plus a 30-day grace period. One percent (N=5,625) of the study population filled two prescriptions of the same SSRI at treatment initiation; we assumed the two prescriptions were taken sequentially, given that the median days’ supply for patients with prescriptions of different doses was seven days (interquartile range [IQR]=7–15 days) for the lower-dose prescriptions and 30 days (IQR=30–30 days) for the higher-dose prescriptions. Therefore, if there was a difference in the dose per day of two index prescriptions, the lower dose was considered the first prescription and the higher dose was considered the second prescription.
Time Periods
Patients were stratified by date of antidepressant initiation into time periods related to three historically relevant dates. [A timeline of relevant events is available as an online supplement to this article.] Period 1, before the onset of heightened concerns about suicide risks of antidepressants, included January 1, 2000, to June 18, 2003, and was further divided into two time periods (January 1, 2000–December 31, 2001, and January 1, 2002–June 18, 2003) to determine whether dosing was stable in the years preceding the initial advisory. Period 2 began June 19, 2003, when the FDA recommended against using paroxetine for the treatment of major depressive disorder among children. Period 3 began on October 15, 2004, when the black-box warning for children under age 18 was announced. Period 4 began on May 2, 2007, when the black-box warning was expanded to persons ages 18–24 and continued until December 31, 2009, when the study ended. Primary comparisons were between periods 1 and 2 and periods 3 and 4. For young adults, an additional comparison of periods 1–3 and period 4 was considered.
Covariates
Baseline patient covariates in the year prior to treatment initiation were collected. Covariates of primary interest included age at treatment initiation, index SSRI agent, provider specialty, and depression diagnosis. Age categories for children (5–9, 10–12, and 13–17) were created on the basis of potential variation in dosing and prescribing practices. Provider specialty was the specialty of the provider associated with the index antidepressant prescription; specialties of interest were psychiatry, psychology, and general practice (including pediatrics and family practice). The remaining specialties were classified as other or, if missing, unknown. A depression diagnosis was defined as having one of the following inpatient or outpatient ICD-9-CM diagnostic codes in the prior year: 296.2x, 296.3x, 298.0x, 300.4x, 309.0x, 309.1x, 311.xx, 293.83, 296.90, and 309.28. Psychiatric and nonpsychiatric morbidities that were prevalent in all age groups were used to help describe the health status of the study cohort (bipolar disorder, ICD–9-CM codes 296.4–296.8; ADHD, 314.x; anxiety disorder, 300.0, 300.2, 300.3; substance use disorder, 291.x, 292.x, 303.x–305.x; cancer, 140.x–208.x; cardiac arrhythmia, 427.x; diabetes, 250.x; cerebrovascular disease, 430.x–437.x; cluster headaches or migraines, 346.x; and seizures, 345.x. Additional covariates described the study cohort.
Analysis
We estimated the proportion of patients initiating an SSRI with a low dose before and after the 2004 FDA black-box warning as well as the change in the percentage and the associated Wald 95% confidence interval (CI). Results were evaluated by period of initiation and age group and were further stratified by SSRI agent to assess variations in prescribing practices around dosing by agent (paroxetine was associated with the initial heightened warnings and fluoxetine was the only FDA-approved SSRI for treatment of major depressive disorder among children at the time of the black-box warning [
1,
4]). Results were also stratified by presence of a depression diagnosis in the year before SSRI initiation and by prescribing provider type. Provider specialty stratification was restricted to psychiatry versus general practice because we cannot be certain of the psychology service provider makeup in the data source given prescribing rights for psychologists were limited to two states during the study period. Two sensitivity analyses were conducted in addition to the sensitivity analysis altering the paroxetine low-dose definition for children 13–17 years: one assumed that the 1% of patients who filled two antidepressant prescriptions at initiation took the prescriptions concurrently rather than sequentially. For example, the initial dose per day for a patient with two initial prescriptions for 10 and 30 mg, respectively, was considered 40 mg/day rather than 10 mg/day. The other sensitivity analysis included the .6% of patients who were excluded from primary analyses because of invalid initial dose values (thought to be entry error). Analyses were conducted in SAS, version 9.3.
Results
Between 2000 and 2009, a total of 51,948 children, 51,653 young adults, and 395,550 adults initiated an SSRI. The majority of children initiated treatment with sertraline (38%) or fluoxetine (35%) compared with citalopram (15%) or paroxetine (11%) (
Table 1). A psychiatrist wrote the initial prescription for 21% of children, 10% of young adults, and 5% of adults. Half of children, 45% of young adults, and 33% of adults had a depression diagnosis in the year prior to SSRI initiation, and 29% of children, 28% of young adults, and 22% of adults had an anxiety diagnosis.
Initial Dose Per Day
Overall, 25% (N=1,694) of children ages 5–9, 10% (N=884) of children ages 10–12, 31% (N=11,165) of children ages 13–17, 21% (N=11,087) of young adults, and 19% (N=76,808) adults initiated an SSRI with a low dose. The proportion of patients initiating with a low dose increased over time (
Figure 1). For children ages 10–12 and 13–17, the increase in the proportion of patients initiating with a low dose began during period 2, the period immediately following the initial paroxetine advisory, and continued thereafter. The proportion of the youngest children (ages 5–9), young adults, and adults initiating SSRI treatment with a low dose increased slightly during period 1, stabilized for young adults and adults in period 3, and further increased among young adults in period 4, after the 2007 black-box expansion.
The increase in the proportion of patients initiating an SSRI with a low dose after the 2004 black-box warning compared with prior to the warning was most prominent among children ages 13–17 (37% versus 17%), a relative increase of 116% (
Table 2). Results were essentially unchanged in the sensitivity analysis that assumed patients with two index antidepressant prescriptions took them concurrently and in the analysis that included patients with invalid initial dose values.
Patients with a depression diagnosis experienced a greater percentage-point change in the proportion initiating an SSRI with a low dose after the 2004 black-box warning compared with patients with no depression diagnosis, and this was true among children (18% versus 14%), young adults (7% versus 5%), and adults (4% versus 2%) (
Table 2). The percentage-point change in the proportion of patients initiating an SSRI with a low dose after the 2004 black-box warning was higher among children with a service provider whose specialty was psychiatry (18 percentage points), compared with children with a provider in general practice (14 percentage points).
The proportion of children ages 13–17 who initiated an SSRI with a low dose differed across agents, ranging from 2% (N=66 of 4,037) among paroxetine initiators to 47% (N=5,987 of 12,779) among fluoxetine initiators, but there was much less variation across agents among children ages 5–12 (13%−19%). However, using the alternative definition of low-dose paroxetine, the sensitivity analysis for children ages 13–17 indicated that 39% (N=874 of 2,270) initiated paroxetine with a low dose before the 2004 warning compared with 52% (N=923 of 1,767) after the warning. When the sensitivity analysis for children ages 13–17 considered all agents, including the alternative definition of low-dose paroxetine, there was an increase of 15 percentage points (CI=14–16) in the proportion of children who initiated with a low dose of any SSRI after the 2004 black-box warning (40%) compared with before the warning (25%).
Dose Augmentation
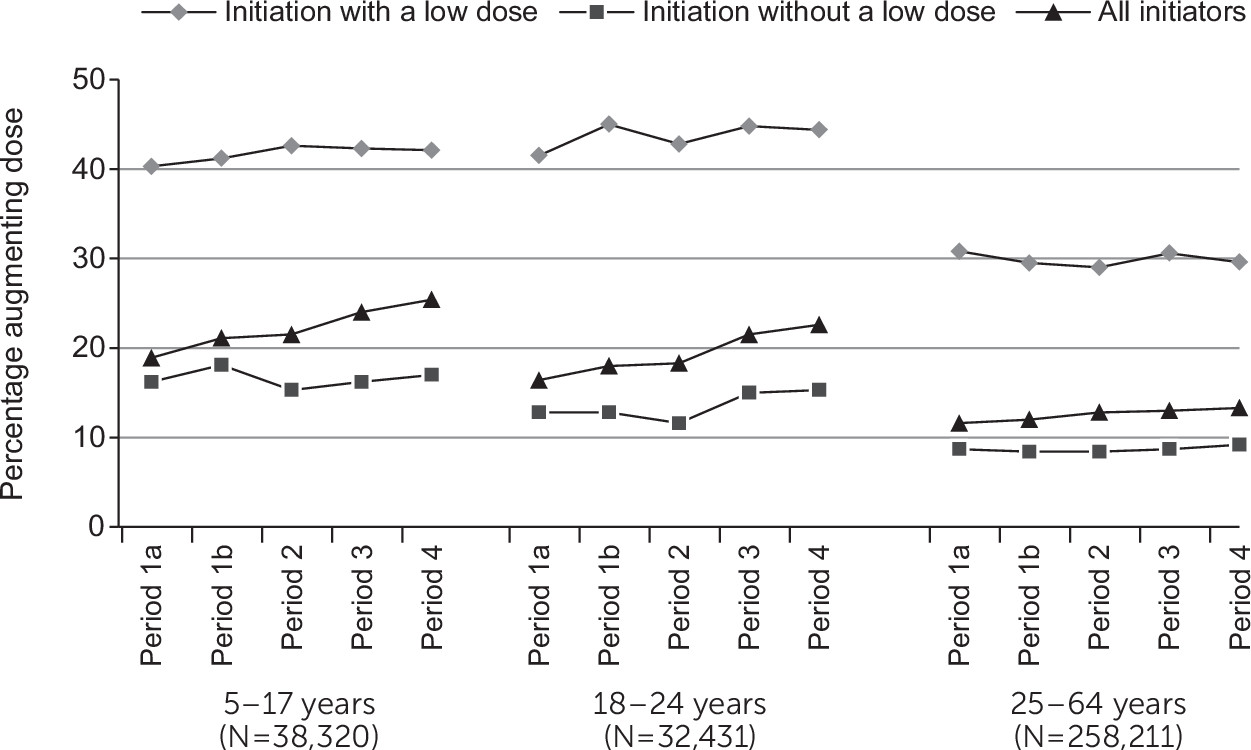
Overall, 74% of children (range [across periods 1–4] 73%−74%), 63% of young adults (range 62%−65%), and 65% of adults (range 64%−69%) filled a second SSRI prescription for the same agent. Of those with a second prescription, the dose was augmented among 980 of 4,972 (20%) children ages 5–9; 1,530 of 7,158 (21%) children ages 10–12; 6,538 of 26,190 (25%) children ages 13–17; 6,809 (21%) young adults; and 33,262 (13%) adults. Patients who initiated an SSRI with a low dose were more likely to subsequently augment the dose compared with patients who did not initiate with a low dose (
Figure 2). Stratified by initial dose, the proportion of patients augmenting the dose on the second fill remained stable across the four time periods (
Figure 2).
Discussion
Overall, we saw an increase in the proportion of children and young adults initiating an SSRI with a low dose after the 2004 black-box warning, compared with the period before the warning, with minimal changes in adults. The change in prescribing practices surrounding dosing was not universal, given that by study’s end, the majority of children who initiated SSRIs did not do so with a low dose. The black-box warning and expansion did not mention dosing, however, and warnings with specific reference to dosing might have a larger impact on dose-related prescribing practices. The increased proportion of children and young adults augmenting the dose on the second fill after the 2004 warning was accounted for by the increase in low-dose initiators.
The largest increase in the proportion of patients who initiated an SSRI with a low dose after the 2004 black-box warning occurred among children ages 13–17. This may be a result of increased concern about suicide in this age group compared with younger children, given that intentional self-harm is more common in this age group (
39,
40). Our finding that a lower proportion of children ages 10–12, compared with children in other age groups, initiated treatment with a low dose (10% versus 25%−31%) during the study period is likely due to changes in our definition of low dose for citalopram, fluoxetine, and sertraline when children turn 13. Our definition of low dose for these agents reflects the fact that a dose considered low for 13- to 17-year-olds may not be considered low among younger children. The oldest children in the 5–12 age group, therefore, were more likely to initiate with a dose that would be considered low for children ages 13–17 but not for children in their age group.
The change in the proportion of children initiating treatment with a low dose was slightly more pronounced for children prescribed antidepressants by psychiatrists versus general practitioners, which may be a result of differential familiarity with treatment guidelines, the black-box warnings, or SSRIs themselves. Our finding that the proportion of children initiating with a low dose varied by SSRI agent may be due in part to the definition of low dose used in primary analyses but may also be related to variations in drug formulation that make it harder to prescribe lower doses—for example, paroxetine CR is not available in doses less than 12.5 mg/day. Given the availability of paroxetine IR and other SSRIs, low doses of other SSRIs could have been prescribed if desired.
The official announcement of the decision to add the black-box warning to all antidepressants was made more than one year after the FDA issued its initial advisory on the possible increased suicidality risk associated with paroxetine. The interval between the initial warning (June 2003) and the formal black-box warning (October 2004) was marked by media coverage on the risk of suicidality associated with pediatric antidepressant use (
41). Consistent with prior research that described decreases in antidepressant prescribing beginning before the 2004 warning (
6,
7,
11,
14,
15), we observed a change in prescribing practices during period 2, especially among children ages 13–17. The proportion of patients in this age group who initiated an SSRI with a low dose was stable during period 1 and doubled in period 2, after the paroxetine advisory.
The black-box warning did not expand to patients 18–24 years of age until 2007. The proportion of patients ages 18–24 initiating an SSRI with a low dose increased then stabilized after the 2004 black-box warning then increased again after the warning was expanded. The changes we observed in the proportion of patients initiating low-dose therapy in period 2 suggest that the initial advisory on the potential suicidality associated with paroxetine use among children might have influenced prescribing among 18- to 24-year-olds. There was a similar increase in the proportion of adults (ages 25–64) initiating an SSRI with a low dose in period 2. These findings of increases in initiation of SSRIs with low doses among patients >18 years of age after the initial advisory may be consistent with spillover effects that have been observed previously (
42). For example, following a 2005 black-box warning regarding the risk of use of second-generation antipsychotics among elderly patients with dementia, modest decreases in use were seen among elderly patients without dementia (
43). In a similar fashion, substantial decreases in antidepressant prescribing among children related to FDA communications (
6,
7,
10,
14,
15,
18,
44,
45) were accompanied by modest decreases among adults (
6,
14), even though the communications were focused on persons younger than 25.
Limitations of our study should be considered. The population of antidepressant initiators increased across the study, which is a function of an increase in the size of the data source; consequently, proportions were weighted toward the end of each period. Dose values were dependent on correct data entry in records of dispensed prescriptions, and free samples may have been provided to the patient at treatment initiation, possibly resulting in misclassification of the initial dose. We would not, however, expect these sources of misclassification to differentially affect children and adults across the time periods. Results were limited to patients with continuous insurance enrollment who initiated citalopram, fluoxetine, paroxetine, or sertraline and who had a measurable dose. Last, the working definition we adopted for low dose was based on available product labels and guidelines and did not account for treatment indications or patient weight.
Conclusions
Results from this descriptive study indicate that the proportion of commercially insured children initiating an SSRI with a low dose was higher in the period after the 2004 FDA warning on the risk of suicidality among children. An increase in the proportion of children who started an SSRI with a low dose was apparent after the initial advisory in 2003. Given recent findings that the dose of the antidepressant may be associated with an increased risk of self-harm (
27,
28), as well as AACAP guidelines indicating that children and adolescents with depressive disorders should initiate antidepressant treatment with a low dose (
19), our results suggest that prescribing practices surrounding SSRI dosing among children improved following the black-box warnings but still fall short of guidelines.